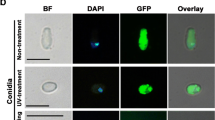
Summary
A homologue of the geneTOR2 fromSaccharomyces cere isiae has been found in the arbuscular mycorrhizal (AM) fungusGlomus mosseae (BEG 12) during a differential RNA display experiment. Further downstream sequence was obtained by a nested-PCR approach. Eight introns were found in 2.6 kb sequence. The fragment encodes a putative protein with high homology (53% identity) to the C terminus ofS. cere isiae TOR2 and its homologues inSchizosaccharomyces pombe and humans. The gene was namedGmTOR2. The expression of the gene was studied by reverse transcriptase-polymerase chain reaction and it was found to be expressed at a relatively high level during all the different life cycle stages of the AM fungus. TOR2 is known to be involved in the control of the cell cycle inS. cere isiae and the organization of the actin cytoskeleton in response to nutrients. The anti-inflammatory drug rapamycin, known to interfere with the role of TOR2 controlling the arrest of the cell cycle in G1 but not with its signalling to the actin cytoskeleton, was found to decrease hyphal growth ofG. mosseae sporocarps but not to affect spore germination. This result confirms that DNA replication is not needed for germination but during the presymbiotic growth. The immunostaining of germinated sporocarps ofG. mosseae with antibodies against tubulin showed the presence of mitotic spindles in some secondary spores, confirming previous findings of DNA replication during presymbiosis. The possibility that GmTOR2 controls the cell cycle arrest in AM fungi in the absence of the plant as a response to nutrient starvation is discussed.
Similar content being viewed by others
Abbreviations
- AM:
-
fungi arbuscular mycorrhizal fungi
- DDRT:
-
differential
- RNA:
-
display
References
Aström H, Giovannetti M, Raudaskoski M (1994) Cytoskeletal components in the arbuscular mycorrhizal fungusGlomus mosseae. Mol Plant Microbe Interact 7: 309–312
Bago B, Zipfel W, Williams RM, Chamberland H, Lafontaine JG, Webb WW, Piche Y (1998) In vivo studies on the nuclear behavior of the arbuscular mycorrhizal fungusGigaspora rosea grown under axenic conditions. Protoplasma 203: 1–15
Barker SJ, Stummer B, Gao L, Dispain I, Oconnor PJ, Smith SE (1998) A mutant inLycopersicon esculentum Mill, with highly reduced VA mycorrhizal colonization: isolation and preliminary characterisation. Plant J 15: 791–797
Bauer D, Müller H, Reich J, Riedel H, Ahrenkiel V, Warthoe P, Strauss M (1993) Identification of differentially expressed mRNA species by an improved display technique (DDRT-PCR). Nucleic Acids Res 21: 4272–4280
Bécard G, Pfeffer PE (1993) Status of nuclear division in arbuscular mycorrhizal fungi during in vitro development. Protoplasma 174: 62–68
—, Douds DD, Pfeffer PE (1992) Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl Environ Microbiol 58: 821–825
Beilby JP (1983) Effects of inhibitors on early protein, RNA, and lipid synthesis in germinating vesicular-arbuscular mycorrhizal fungal spores ofGlomus caledonius. Can J Microbiol 29: 596–601
Bianciotto V, Bonfante P (1993) Evidence of DNA replication in an arbuscular mycorrhizal fungus in the absence of the host plant. Protoplasma 176: 100–105
—, Barbiero G, Bonfante P (1995) Analysis of cell cycle in an arbuscular mycorrhizal fungus by flow cytometry. Protoplasma 188: 161–169
Bierer BE, Mattila PS, Standaert RF, Herzenberg LA, Burakoff SJ, Crabtree G, Schreiber SL (1990) Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci USA 87: 9231–9235
Bonfante P, Perotto S (1995) Transley review no. 82: strategies of arbuscular mycorrhizal fungi when infecting host plants. New Phytol 130: 3–21
Bradbury SM, Peterson RL, Bowley SR (1991) Interactions between three alfalfa nodulation genotypes and two glomus species. New Phytol 119: 115–120
Cardenas ME, Heitman J (1995) FKBP12-rapamycin target TOR2 is a vacuolar protein with an associated phosphatidylinositol-4 kinase activity. EMBO J 14: 5892–5907
Chen J, Zheng X-F, Brown EJ, Schreiber SL (1995) Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKB12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA 92: 4947–4951
Due G, Trouvelot A, Gianinazzi-Pearson V, Gianinazzi S (1989) First report of non-mycorrhizal mutants (myc-) obtained in pea (Pisum sati um L.) and fababean (Vicia faba L.). Plant Sci 60: 215–222
Fischer R, Timberlake WE (1995)Aspergillus nidulans apsA (Anucleate Primary Sterigmata) encodes a coiled-coil protein for nuclear positioning and completion of asexual development. J Cell Biol 128: 485–498
Franken P, Gianinazzi-Pearson V (1996) Phage cloning of ribosomal RNA genes from the arbuscular mycorrhizal fungiGlomus mosseae andScutellospora castanea. Mycorrhiza 6: 167–173
—, Gnädinger F (1994) Analysis of parsley arbuscular endomycorrhiza: infection development and mRNA levels of defense-related genes. Mol Plant Microbe Interact 7: 612–620
Gery S, Lavi S (1997) Purification and cloning of differential display products. Biotechniques 23: 198–202
Giovannetti M, Avio L, Sbrana C, Citernesi AS (1993) Factors affecting appressorium development in the vesicular-arbuscular mycorrhizal fungusGlomus mosseae. New Phytol 123: 115–122
Harrison MJ (1999) Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol Plant Mol Biol 50: 361–389
Heitman J, Movna NR, Hall MN (1991) Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253: 905–909
Helliwell SB, Howald I, Barbet N, Hall MN (1998) TOR2 is part of two related signaling pathways coordinating cell growth inSaccharomyces cere isiae.. Genetics 148: 99–112
Hepper CM (1979) Germination and growth ofGlomus caledonius spores: the effects of inhibitors and nutrients. Soil Biol Biochem 2: 269–277
Karpova TS, McNally JG, Moltz SL, Cooper JA (1998) Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J Cell Biol 142: 1501–1517
Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Rao Movva N, Hall MN (1993) Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 73: 585–596
Logi C, Sbrana C, Giovannetti M (1998) Cellular events involved in survival of individual arbuscular mycorrhizal symbionts growing in the absence of the host. Appl Environ Microbiol 64: 3473–3479
Lorenz MC, Heitman J (1995) TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem 270: 27531–27537
MacDonald RM, Lewis M (1978) The occurrence of some acid phosphatases and dehydrogenases in the vesicular-arbuscular mycorrhizal fungusGlomus mosseae. New Phytol 80: 135–141
Martin-Laurent F, Van Tuinen D, Dumas-Gaudot E, Gianinazzi-Pearson V, Gianinazzi S, Franken P (1997) Differential display analysis of RNA accumulation in arbuscular mycorrhiza of pea and isolation of a novel symbiosis-regulated plant gene. Mol Gen Genet 256: 37–44
Mosse B (1962) The establishment of vesicular-arbuscular mycorrhiza under aseptic conditions. J Gen Microbiol 27: 509–520
Nagahashi G, Douds DD Jr (1997) Appressorium formation by AM fungi on isolated cell walls of carrot roots. New Phytol 136: 299–304
Powers T, Walter P (1999) Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway inSaccharomyces cere isiae. Mol Biol Cell 10: 987–1000
Requena N (1998) Mycorrhizal interactions in the rhizosphere. In: Varma A (ed) Microbes: for health, wealth and sustainable environment. Malhotra Publishing House, New Delhi, pp 725–738
—, Füller P, Franken P (1999)GmFOX2 a highly conserved gene from endomycorrhiza is down-regulated during pre-symbiosis in response to rhizobacteria. Mol Plant Microbe Interact 12: 934–942
Schmidt A, Kunz J, Hall MN (1996) TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci USA 93: 13780–13785
—, Beck T, Koller A, Kunz J, Hall MN (1998) The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J 17: 6924–6931
Thomas G, Hall MN (1997) TOR signalling and control of cell growth. Curr Opin Cell Biol 9: 782–787
Vilella-Bach M, Nuzzi P, Fang Y, Chen J (1999) The FKBP12-rapamycin-binding domain is required for FKBP12-rapamycin-associated protein kinase activity and G1 progression. J Biol Chem 274: 4266–4272
Wegel E, Schauser L, Sandal N, Stougaard J, Parniske M (1998) Mycorrhiza mutants ofLotus japonicus define genetically independent steps during symbiotic infection. Mol Plant Microbe Interact 11: 933–936
Zaragoza D, Ghavidel A, Heitman J, Schultz MC (1998) Rapamycin induces the GO program of transcriptional repression in yeast by interfering with the TOR signaling pathway. Mol Cell Biol 18: 4463–4470
Zézé A, Dulieu H, Gianinazzi-Pearson V (1994) DNA cloning and screening of a partial genomic library from an arbuscular mycorrhizal fungus,Scutellospora castanea. Mycorrhiza 4: 251–254
Zheng XF, Florentine D, Chen J, Crabtree GR, Schreiber SL (1995) TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell 82: 121–130
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Requena, N., Mann, P. & Franken, P. A homologue of the cell cycle check point TOR2 fromSaccharomyces cerevisiae exists in the arbuscular mycorrrhizal fungusGlomus mosseae . Protoplasma 212, 89–98 (2000). https://doi.org/10.1007/BF01279350
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279350