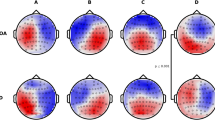
Summary
The only available functional neuroimaging methods reaching the time resolution of human information processing are EEG and MEG. Since spectral analysis implies analysis of longer time epochs, the high temporal resolution of EEG is partly lost. By dividing the EEG in the time-domain into segments of similar spatial distribution on the scalp (microstates) it has been possible to assess patterns of neuronal activity representing the information process currently performed by the brain. In the present study alterations of EEG microstates in subjective (n=31) and objective (n=38) memory impairment as well as in probable Alzheimer disease (DAT: n=64) compared to healthy controls (n=21) were investigated. The main findings were reduced segment durations and a more anterior center of gravity of the microstate topography in DAT. With more pronounced cognitive dysfunction larger window sizes were found. Shorter microstates and larger windows reflect more rapidly changing spatial activation patterns, and are interpreted as an impaired capability to establish stable brain states necessary for normal brain function. The anteriorization of the microstates is consistent with results in the frequency domain and may reflect neuropathological changes in DAT.
Similar content being viewed by others
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorder, 4th edn. American Psychiatric Association, Washington DC, pp 1–142
Barlow JS (1985) Methods of analysis of nonstationary EEGs, with emphasis on segmentation techniques: a comparative review. J Clin Neurophysiol 2: 267–304
Braak H, Braak E, Bohl J (1993) Staging of Alzheimer-related cortical destruction. Eur Neurol 33: 403–408
Brun A, Gustafson L (1976) Distribution of cerebral degeneration in Alzheimer's disease. Arch Psychiatr Nervenkr 223: 15–33
Debecker J, Desmedt JE (1970) Maximum capacity for sequential one-bit auditory decisions. J Exp Psychol 83: 366–372
Dierks T, Maurer K (1990) Reference-free evaluation of auditory evoked potentials — P300 in aging and dementia. In: Dostert P, Riederer P, Strolin Benedetti M, Roncucci R (eds) Early markers in Parkinson's and Alzheimer's disease. Springer, Wien New York, pp 197–208 (New Vistas in Drug Research, vol 1)
Dierks T, Perisic I, Froelich L, Ihl R, Maurer K (1991) Topography of quantitative EEG in dementia of Alzheimer Type: relation to severity of dementia. Psychiatry Res — Neuroimaging 40(3): 181–194
Dierks T, Ihl R, Frölich L, Maurer K (1993) Dementia of Alzheimer type (DAT): effects on the spontaneous EEG described by dipole sources. Psychiatry Res — Neuroimaging 50: 151–162
Duara R, Grady C, Haxby J, Sundaram M, Cutler NR, Heston L, Moore A, Schlageter N, Larson S, Rapoport SI (1986) Positron emission tomography in Alzheimer's disease. Neurology 36: 879–887
Duffy FH, McAnulty G, Albert MS (1995) Temporoparietal electrophysiological differences characterize patients with Alzheimer's disease: a split-half replication study. Cerebral Cortex 3: 215–221
Fazekas F (1990) Neuroimaging of dementia. Curr Opin Neurol Neurosurg 3: 103–107
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198
Gath I, Bar On E (1983) Classical sleep stages and the spectral content of the EEG signal. Int J Neurosci 22: 147–155
Gevins AS, Schaffer RE, Doyle JC, Cutillo BA, Tannehill RS, Bressler SL (1983) Shadows of thought: shifting lateralization of human brain electrical patterns during brief visuomotor task. Science 220: 97–99
Grochulski W, Penczek P (1986) Syntactic analysis of the epileptic electroencephalogram. Int J Biomed Comput 19: 219–234
Ihl R, Dierks T, Frölich L, Maurer K (1993) Segmentation of the spontaneous EEG in dementia of the Alzheimer type. Neuropsychobiology 27: 231–236
Jelic V, Shigeta M, Julin P, Almkvist O, Winblad B, Wahlund LO (1996) Quantitative electroencephalography power and coherence in Alzheimer's disease and mild cognitive impairment. Dementia 7: 314–323
Lehmann D (1984) EEG assessment of brain activity: spatial aspects, segmentation and imaging. Int J Psychophysiol 1: 267–276
Lehmann D (1992) Brain electrical fields and brain functional states. In: Friedrich R, Wunderlin A (eds) Evolution of dynamical structures in complex systems. Springer, Berlin Heidelberg New York Tokyo, pp 235–248
Lehmann D, Skrandies W (1980) Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol 48: 609–621
Lehmann D, Ozaki H, Pal I (1987) EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalogr Clin Neurophysiol 67: 271–288
McClelland JL, Rumelhart DE, Hinton GE (1986) The appeal of parallel distributed processing. In: Rumelhart DE, McClelland JL (eds) Parallel distributed processing, vol 1. Massachusetts Institute of Technology Press, Cambridge MA, pp 4–44
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer's disease: report of NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology 34: 939–944
Mesulam MM (1990) Large-scale neurocognitive networks and distributed processing for attention, language and memory. Ann Neurol 28: 597–613
Mohr E, Cox C, Williams J, Chase TN, Fedio P (1990) Impairment of central auditory function in Alzheimer's disease. J Clin Exp Neuropsychol 12: 235–246
Murphy DG, DeCarli CD, Daly E, Gillette JA, McIntosh AR, Haxby JV, Teichberg D, Schapiro MB, Rapoport SI, Horwitz B (1993) Volumetric magnetic resonance imaging in men with dementia of the Alzheimer type: correlations with disease severity. Biol Psychiatry 34: 612–621
Nebes RD, Brady CB (1992) Generalized cognitive slowing and severity of dementia in Alzheimer's disease: implications for the interpretation of response-time data. J Clin Exp Neuropsychol 14: 317–326
Nordberg A (1993) Clinical studies in Alzheimer patients with positron emission tomography. Behav Brain Res 57: 215–224
Parasuraman R, Greenwood PM, Haxby JV, Grady CL (1992) Visuospatial attention in dementia of the Alzheimer type. Brain 115: 711–733
Praetorius HM, Bodenstein G, Creutzfeldt OD (1977) Adaptive segmentation of EEG records: a new approach to automatic EEG analysis. Electroencephalogr Clin Neurophysiol 42: 84–94
Rediess S, Caine ED (1996) Aging, cognition and DSM-IV. Aging, Neuropsychology and Cognition 3: 105–117
Soininen H, Partanen J, Paakonen A, Koivisto E, Riekkinen PJ (1991) Changes in absolute power values of EEG spectra in the follow-up of Alzheimer's disease. Acta Neurol Scand 83: 133–136
Stevens A, Günther W, Lutzeberger W, Bartels M, Müller N (1996) Abnormal topography of EEG microstates in Gilles de la Tourette syndrome. Clin Neurosci 246: 310–316
Strik WK (1995) Short and sustained brain electrical microstates and their relevance for cognitive processes. Riv Pat Nerv Ment 3: 17–28
Strik W, Lehmann D (1993) Data-determined window size and space-oriented segmentation of spontaneous EEG map series. Electroencephalogr Clin Neurophysiol 87: 169–174
Strik WK, Dierks T, Lehmann D (1994) Spatial configuration of microstates in the spontaneous EEG of residual schizophrenics. Brain Topogr 6: 252
Strik WK, Dierks T, Becker T, Lehmann D (1995) Larger topographical variance and decreased duration of brain electric microstates in depression. J Neural Transm [Gen Sect] 99: 213–222
Wackermann J, Lehmann D, Michel CM, Strik WK (1993) Adaptive segmentation of spontaneous EEG map series into spatially defined microstates. Int J Psychophysiol 14: 269–283
Wahlund LO, Andersson-Lundman G, Basun H, Almkvist O, Björksten KS, Saaf J, Wetterberg L (1993) Cognitive functions and brain structures: a quantitative study of CSF volumes on alzheimer patients and healthy control subjects. Magn Reson Imaging 11: 169–174
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dierks, T., Jelic, V., Julin, P. et al. EEG-microstates in mild memory impairment and Alzheimer's disease: possible association with disturbed information processing. J. Neural Transmission 104, 483–495 (1997). https://doi.org/10.1007/BF01277666
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01277666