Summary
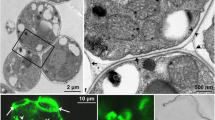
Monokaryotic haustoria (M-haustoria) ofUromyces vignae inVigna sinensis cells are surrounded by an extrahaustorial matrix (ema) and the invaginated host plasmalemma, the extrahaustorial membrane (ehrn). The ema was characterized with antibodies against components of the plant cell wall; the ema contained hydroxyproline-rich glycoproteins and arabinogalactans/arabinogalactan proteins, both at a higher concentration close to the ehm. Haustoria with large vacuoles had the ema encased by additional layers. An electron-translucent inner layer deposited on top of the ema contained arabinogalactans/arabinogalactan proteins, hydroxyproline-rich glycoproteins, and callose. The inner layer was surrounded by an electron-translucent middle layer with numerous dark inclusions, rich in pectin and fucose bound to xyloglucans. Finally, a more electron-dense outer layer containing arabinogalactans/arabinogalactan proteins and hydroxyproline-rich glycoproteins encased the whole structure. These polysaccharides, with the exception of callose and un-esterified pectin, were also found in the plant Golgi apparatus. The polysaccharides were synthesized in the trans Golgi cisternae and secreted into the host-parasite interface. The secretory events seem to be coupled to endocytosis since numerous coated pits were found on the ehm too. The pits were elongated, sometimes formed tubules and the coat reacted with an antibody against plant clathrin. Our results suggest intensive membrane recycling around haustoria, together with the secretion of cell wall material, which in the case of more or less vacuolated haustoria seems to be responsible for encasement
Similar content being viewed by others
Abbreviations
- AG/AGP:
-
arabinogalactans and arabinogalactan proteins
- BSA:
-
bovine serum albumin
- ehm:
-
extrahaustorial membrane
- ema:
-
extrahaustorial matrix
- HRGP2b :
-
hydroxyproline rich glycoproteins
- M-haustorium:
-
monokaryotic haustorium
- TBS:
-
tris buffered saline
References
Al-Khesraji TO, Lösel DM (1981) The fine structure of haustoria, intracellular hyphae and intercellular hyphae ofPuccinia poarum. Physiol Plant Pathol 19: 301–311
Bofante-Fasolo P, Peretto R, Perotto S (1992) Cell surface interactions in endomycorrhizal symbiosis. In: Callow JA, Green JR (eds) Perspectives in plant cell recognition. Cambridge University Press, Cambridge, pp 239–255
Bradley DJ, Wood EA, Larkins AP, Galfre G, Butcher GW, Brewin NJ (1988) Isolation of monoclonal antibodies reacting with peribacteroid membranes and other components of pea root nodules containingRhizobium leguminosarium. Planta 173: 149–160
Cheong JJ, Hahn M (1991) A specific high-affinity binding site for the hepta-β-glucoside elicitor exists in soybean membranes. Plant Cell 3: 137–147
Cluett EB, Wood SA, Banta M, Brown WJ (1993) Tubulation of Golgi membranes in vivo and in vitro in the absence of Brefeldin-A. J Cell Biol 120: 15–24
Cohen Y, Eyal H, Hanania J (1990) Ultrastructure, autofluorescence, callose deposition and lignification in susceptible and resistant muskmelon leaves infected with powdery mildew fungusSphaerotheca fuliginea. Physiol Mol Plant Pathol 36: 191–204
Cosio FG, Frey T, Ebel J (1990) Solubilization of soybean membrane binding sites for fungal β-glucans that elicit phytoalexin accumulation. FEBS 264: 235–238
Ebel J, Cosio FG (1994) Elicitors of plant defense responses. Int Rev Cytol 148: 1–36
Fowke LC, Tanchak MA, Galway ME (1991) Ultrastructural cytology of the endocytotic pathway in plants. In: Hawes CR, Evans DE, Coleman JOD (eds) Endocytosis and vesicle traffic in plants. Cambridge University Press, Cambridge, pp 15–40
Gold RE, Littlefield LH, Statler ED (1979) Ultrastructure of the pycnial and aecial stages ofPuccinia recondita. Can J Bot 57: 74–86
Grief G, Shaw PJ (1987) Assembly of cell wall glycoproteins ofChlamydomonas reinhardtii: oligosaccharides are added in the medial and trans Golgi compartments. Planta 171: 302–312
Harder DE (1979) Comparative ultrastructure of the haustoria in uredial and pycnial infections ofPuccinia coronata avenae. Can J Bot 56: 214–224
—, Chong J (1991) Rust haustoria. In: Mendgen K, Lesemann DE (eds) Electron microscopy of plant pathogens. Springer, Berlin Heidelberg New York Tokyo, pp 235–250
—, Rohringer RJSD, Kim WK, Chong J (1978) Electron microscopy of susceptible and resistant near-isogenic (sr6/Sr6) lines of wheat infectedPuccinia graminis tritici. I. The host-pathogen interface in the compatible (sr6/P6) interaction. Can J Bot 56: 2955–2966
Heath MC (1971) Haustorial sheath formation in cowpea leaves immune to rust infection. Phytopathology 61: 383–388
— (1982) Host defence mechanisms against infection by rust fungi. In: Scott KJ, Chakravorty AK (eds) The rust fungi. Academic Press, London, pp 223–245
— (1988) Effect of fungal death or inhibition induced by oxycarboxin or polyoxin D on the interaction between resistant or susceptible bean cultivars and the bean rust fungus. Phytopathology 78: 1454–1462
Holstein SEH, Daneker M, Robinson DG (1994) Identification of a β-type adaptin in plant clathrin-coated vesicles. J Cell Sci 107: 945–953
Kauss H (1994) Callose synthesis. In: Smallwood M, Knox P, Bowles DJ (eds) Specialized functions in plant cells. JAI Press, London, pp 609–631
Knauf G, Welter K, Müller M, Mendgen K (1989) The haustorial host-parasite interface in rust-infected bean leaves after high pressure freezing. Physiol Mol Plant Pathol 34: 519–530
Knox JP, Linstead PJ, King J, Cooper C, Roberts K (1990) Pectin esterification is spatially regulated within cell walls and between developing tissues of root apices. Planta 181: 512–521
— (1992) Molecular probes for the plant cell surface. Protoplasma 167: 1–9
Larous L, Lösel D (1993) Strategies of pathogenicity in monokaryotic and dikaryotic phases of rust fungi, with special reference to vascular infection. Mycol Res 97: 415–420
Littlefield LJ, Heath MC (1979) Ultrastructure of rust fungi. Academic Press. New York
Low PS, Chandra S (1994) Endocytosis in plants. Annu Rev Plant Physiol Plant Mol Biol 45: 609–631
Mazau D, Rumeau D, Esquerré-Tugayé M-T (1988) Two different families of hydroxyproline-rich glycoproteins in melon callus. Plant Physiol 86: 540–546
Meikle PJ, Bonig I, Hoogenraad NJ, Clarke AE, Stone BA (1991) The location of (1–3)-β-glucans in the walls of pollen tubes ofNicotiana alata using a (1-3)-β-specific monoclonal antibody. Planta 185: 1–8
Mendgen K, Welter K, Scheffold F, Knauf-Beiter G (1991) High pressure freezing of rust infected plant leaves. In: Mendgen K, Lesemann DE (eds) Electron microscopy of plant pathogens. Springer, Berlin Heidelberg New York Tokyo, pp 31–42
Moore PJ, Swords KMM, Lynch MA, Staehelin LA (1991) Spatial organization of assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J Cell Biol 112: 589–602
Oparka KJ, Wright KM, Murant EA, Allan EJ (1993) Fluid-phase endocytosis: do plants need it? J Exp Bot 44: 247–255
Pennell RI (1992) Cell surface arabinogalactan proteins, arabinogalactans and plant development. In: Callow JA, Green JR (eds) Perspectives of plant cell recognition. Cambridge University Press, Cambridge, pp 105–121
—, Knox JP, Scofield GN, Selvendran RR, Roberts K (1989) A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol 108: 1967–1977
Puhlmann J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AG, Hahn MG (1994) Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1-2) linked-fucosyl-containing epitope. Plant Physiol 104: 699–710
Rijkenberg FHJ, Truter S (1973) Haustoria and intracellular hyphae in the rusts. Phytopathology 63: 281–286
Roberts K (1990) Structures at the plant cell surface. Curr Opin Cell Biol 2: 920–928
Robinson DG, Hillmer S (1990 a) Endocytosis in plants. Physiol Plant 79: 94–104
— — (1990 b) Coated pits. In: Larsson C, Møller IM (eds) The plant plasma membrane. Springer, Berlin Heidelberg New York Tokyo, pp 233–255
Schmidt WE. Ebel J (1987) Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybeanGlycine max. Proc Natl Acad Sci USA 84: 4177–4121
Sherrier DJ, Vandenbosch KA (1994) Secretion of cell wall oligosaccharides in Vicia root hairs. Plant J 5: 185–195
Smythe E, Warren G (1991) The mechanism of receptor-mediated endocytosis. Eur J Biochem 202: 689–699
Stark-Urnau M, Mendgen K (1993) Differentiation of aecidiospore- and uredospore-derived infection structures on cowpea leaves and on artificial surfaces byUromyces vignae. Can J Bot 71: 1236–1242
Steer MW (1988) Plasma membrane turnover in plants. J Exp Bot 39: 987–996
Woods AM, Gay JL (1987) The interface between haustoria ofPuccinia poarum (monokaryon) andTussilage farfara. Physiol Mol Plant Pathol 30: 167–185
Zhang GF, Staehelin LA (1992) Functional compartimentation of the Golgi apparatus of plant cells. Immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99: 1070–1083
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stark-Urnau, M., Mendgen, K. Sequential deposition of plant glycoproteins and polysaccharides at the host-parasite interface ofUromyces vignae andVigna sinensis . Protoplasma 186, 1–11 (1995). https://doi.org/10.1007/BF01276929
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276929