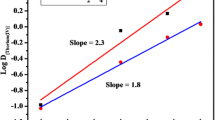
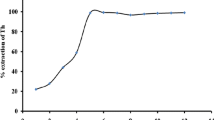
Summary
The solvent extraction separation of thorium with tri-n-octyl-phosphine oxide was studied. Thorium was determined in gross quantities of uranium compounds using a two-cycle solvent extraction procedure in the presence of EDTA. Uranium was shown to be preferentially extracted by TOPO from a 2M nitrate solution. In addition, it was found that yttrium and the rare earths are extracted by TOPO. Elimination of this interference was accomplished by backwashing with a fresh 2M nitrate wash solution. The methods developed are rapid, selective, and sensitive for the spectrophotometric determination of thorium and yttrium. The systems obey Beer's law under all concentration ranges studied, and by proper choice of final concentration and absorbance cells, the methods can be applied over wide ranges of concentration.
Zusammenfassung
Die Abtrennung von Thorium durch Extraktion mit Tri-n-oktylphos-phinoxid (TOPO) wurde untersucht. In großen Mengen Uranverbindungen wurde Thorium mit Hilfe eines Zweiphasenganges in Gegenwart von ÄDTA extrahiert. Uran wird zuvor mit TOPO aus 2-m Nitratlösung extrahiert. Außerdem werden, wie sich gezeigt hat, Yttrium und die seltenen Erden mit TOPO extrahiert. Diese Störung wurde durch Rückextraktion mit einer frischen 2-m Nitratwaschlösung beseitigt. Die rasche, selektive und empfind-liche Arbeitsweise eignet sich zur spektrophotometrischen Bestimmung von Thorium und Yttrium. Die Lösungen entsprechen in allen untersuchten Konzentrationsbereichen dem Beerschen Gesetz. Bei richtiger Wahl der Endkonzentration und der Absorptionskuvetten kann die Methode für ein breites Konzentrationsgebiet verwendet werden.
Similar content being viewed by others
References
H. Levine and F. S. Grimaldi, U. S. Geol. Survey, Bull. No. 1006, 177 (1954).
C. V. Banks and C. H. Byrd, Analyt. Chemistry25, 416 (1953).
C. V. Banks, D. W. Klingman, and C. H. Byrd, Analyt. Chemistry25, 992 (1953).
P. F. Thomason, M. A. Perry, and W. M. Byerly, Analyt. Chemistry21, 1239 (1949).
E. K. Hyde and J. Tolmach, USAEC Report ANL-4248, February 1949.
O. Menis, D. L. Manning, and G. Goldstein, Analyt. Chemistry29, 1426 (1957).
D. A. Everest and J. V. Martin, Analyst84, 312 (1959).
W. J. Ross and J. C. White, USAEC Report ORNL-2627, November 1958.
W. J. Ross and J. C. White, Analyt. Chemistry31, 1847 (1959).
W. C. Johnson Jr., and M. H. Campbell, Analyt. Chemistry37, 1440 (1965).
J. C. Guyon and B. L. Madison, Analyt. Chemistry39, 1707 (1967).
H. Okada, K. Kaneko, and S. Goseki, Bunseki Kagaku12, 822 (1963).
C. A. Horton and J. G. White, Analyt. Chemistry30, 1779 (1958).
M. J. Cabell, Analyst77, 859 (1952).
V. T. Athavale, L. M. Manajan, N. R. Thakoor, and M. S. Varde, Analyt. Chim. Acta21, 353 (1959).
M. K. Carron, D. L. Skinner, and R. E. Stevens, Analyt. Chemistry27, 1058 (1955).
Author information
Authors and Affiliations
Additional information
Supported by AF-AFOSR—205-65.
Rights and permissions
About this article
Cite this article
Guyon, J.C., Madison, B. Studies on the solvent extraction of thorium. Mikrochim Acta 63, 133–144 (1975). https://doi.org/10.1007/BF01271530
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01271530