Summary
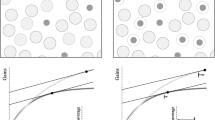
Natural populations live in heterogeneous environments, where habitat variation drives the evolution of phenotypic plasticity. The key feature of population structure addressed in this paper is the net flow of individuals from source (good) to sink (poor) habitats. These movements make it necessary to calculate fitness across the full range of habitats encountered by the population, rather than independently for each habitat. As a consequence, the optimal phenotype in a given habitat not only depends on conditions there but is linked to the performance of individuals in other habitats. We generalize the Euler-Lotka equation to define fitness in a spatially heterogeneous environment in which individuals disperse among habitats as newborn and then stay in a given habitat for life. In this case, maximizing fitness (the rate of increase over all habitats) is equivalent to maximizing the reproductive value of newborn in each habitat but not to maximizing the rate of increase that would result if individuals in each habitat were an isolated population. The new equation can be used to find optimal reaction norms for life history traits, and examples are calculated for age at maturity and clutch size. In contrast to previous results, the optimal reaction norm differs from the line connecting local adaptations of isolated populations each living in only one habitat. Selection pressure is higher in good and frequent habitats than in poor and rare ones. A formula for the relative importance of these two factors allows predictions of the habitat in which the genetic variance about the optimal reaction norm should be smallest.
Similar content being viewed by others
References
Andrewartha, H. G. and Birch L. C. (1954)The Distribution and Abundance of Animals. University of Chicago Press, Chicago, USA.
Bergerud, A. T. (1988) Caribou, wolves and man.Trends Ecol. Evol. 3, 68–72.
Blondel, J., Perret, P., Maistre, M. and Dias, P. (1992) Do harlequin Mediterranean environments function as source sink for Blue Tits (Parus caeruleus L.)?Landscape Ecology 7, 213–9.
Caswell, H. (1989a)Matrix Population Models. Sinauer Associates, Inc., Sunderland, Massachusetts, USA.
Caswell, H. (1989b) Life-history strategies.Ecological Concepts. (J. M. Cherrett, ed.), pp. 285–308. Blackwell Scientific, Oxford, UK.
Charlesworth, B. (1980)Evolution in Age-Structured Populations. Cambridge University Press, Cambridge, UK.
Charlesworth, B. (1990) Optimization models, quantitative genetics, and mutation.Evolution 44: 520–38.
Charnov, E. L. (1986) Life history evolution in a ‘recruitment population’: why are adult mortality rates constant?Oikos 47, 129–34.
Charnov, E. L. (1989) Phenotypic evolution under Fisher's Fundamental Theorem of Natural Selection.Heredity 62, 113–16.
Charnov, E. L. (1990) On evolution of age at maturity and the adult lifespan.J. Evol. Biol. 3: 139–44.
Charnov, E. L., los-den Hartogh, R. L., Jones T. and van dem Assem, J. (1981) Sex ratio evolution in a patchy environment.Nature 289, 27–33.
Connell, J. H. (1961) The influence of interspecific competition and other factors on the distribution of the barnacleChthamalus stellatus.Ecology 42, 710–23.
Daan, S., Dijkstra C. and Tinbergen J. M. (1990) Family planning in the kestrel (Falco tinnunculus): the ultimate control of covariation of laying date and clutch size.Behavior 114, 83–116.
Dhondt, A. A., Adriaensen, F., Matthysen, E. and Kempenaers, B. (1990) Nonadaptive clutch sizes in tits.Nature 348, 723–5.
Emlen, J. M. (1970) Age specificity and ecological theory.Ecology 51, 588–601.
Goodman, D. (1982) Optimal life histories, optimal notation, and the value of reproductive value.Am.Nat. 119, 803–23.
Haldane, J. B. S. (1927) A mathematical theory of natural and artificial selection. Part IV.Proc. Camb. Phil. Soc. 23, 607–15.
Hamilton, W. D. (1966) The moulding of senescence by natural selection.J. Theor. Biol. 12, 12–45.
Hastings, A. (1978) Evolutionarily stable strategies and the evolution of life history strategies: I. Density dependent models.J. Theor. Biol. 75, 527–36.
Houston, A. I. and McNamara, J. M. (1992) Phenotypic plasticity as a state dependent life-history decision.Evol. Ecol. 6, 243–53.
de Jong, G. (1990) Quantitative genetics of reaction norms.J. Evol. Biol. 3, 447–68.
Kozlowski, J. and Uchmanski, J. (1987) Optimal individual growth and reproduction in perennial species with indeterminate growth.Evol. Ecol. 1, 214–30.
Kozlowski, J. and Wiegert, R. G. (1986) Optimal allocation of energy to growth and reproduction.Theor. Pop. Biol. 29, 16–37.
McNamara J. M. (1991). Optimal life histories: a generalization of the Perron-Frobenius Theorem.Theor. Pop. Biol. 40, 230–45.
Pulliam, H. R. (1988) Sources, sinks, and population regulation.Am. Nat. 132, 652–61.
Pulliam, H. R and Danielson, B. J. (1991) Sources, sinks, and habitat selection: a landscape perspective on population dynamics. Am. Nat.137, S50-S66.
Reiter, J. and Le Boeuf, B. J. (1991) Life history consequences of variation in age at primiparity in northern elephant seals.Behav. Ecol. Sociobiol. 28, 153–60.
Rosenzweig, M. L. (1991) Habitat selection and population interactions: the search for mechanism.Am. Nat. 137, S5-S28.
Seger, J. and Brockmann, H. J. (1987) What is bet-hedging?Oxford Surveys in Evolutionary Biology. (P. H. Harvey and L. Partridge, eds) Vol. 4, pp 182–211. Oxford University Press, Oxford, UK.
Schaffer, W. M. (1974) Selection for optimal life histories: the effects of age structure.Ecology 55, 291–303.
Sibly, R. M. and Calow, P. (1986)Physiological Ecology of Animals. Blackwell Scientific, Oxford, UK.
Sinervo, B. (1990) The evolution of maternal investment in lizards: An experimental and comparative analysis of egg size and its effects on offspring performance.Evolution 44, 279–94.
Stearns, S. C. and Crandall, R. E. (1981) Quantitative predictions of delayed maturity.Evolution 35, 455–63.
Stearns, S. C. and Koella, J. (1986) The evolution of phenotypic plasticity in life-history traits: predictions for norms of reaction for age- and size-at-maturity.Evolution 40, 893–913.
Stearns, S. C. and Sage, R. D. (1980) Maladaptation in a marginal population of mosquito fish,Gambusia affinis.Evolution 34, 65–75.
Via, S. and Lande, R. (1985) Genotype-environment interaction and the evolution of phenotypic plasticity.Evolution 39, 505–22.
Wright, S. L., Crawford, C. B. and Anderson, J. L. (1988) Allocation of reproductive effort inMus domesticus: responses of offspring sex ratio and quality to social density and food availability.Behav. Ecol. Sociobiol. 23, 357–65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawecki, T.J., Stearns, S.C. The evolution of life histories in spatially heterogeneous environments: Optimal reaction norms revisited. Evol Ecol 7, 155–174 (1993). https://doi.org/10.1007/BF01239386
Issue Date:
DOI: https://doi.org/10.1007/BF01239386