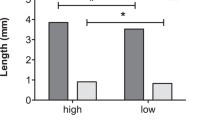
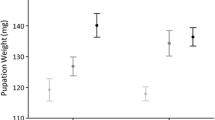
Summary
It has been hypothesized that reproductive character displacement has evolved in mainland Sonora, Mexico populations of cactophilicD. mojavensis due to the presence of a sympatric sibling speciesD. arizonae. In laboratory tests using ancestral Baja California populations and derived, sympatric mainland populations, asymmetrical sexual isolation has been observed among populations ofD. mojavensis where mainland females discriminate against Baja males. Effects of different pre-adult rearing environments on adult mating behaviour were assessed by comparing fermenting cactus tissues like those used in nature for breeding with laboratory media because previous studies have employed synthetic growth media for fly growth and development. Significant behavioural isolation was evident in all cases when larvae were reared on laboratory food, but was non-significant when flies were reared on fermenting cactus, except for the cactus used by most mainland populations, consistent with previous studies. Time to copulation of Baja females was greater than mainland females over all substrates, but male time to copulation did not differ between populations. Time to copulation for both sexes was significantly greater when flies were reared on laboratory food with one exception. The degree of behavioural isolation was weakly correlated with time to copulation across food types (Spearman rank correlation = 0.58,p = 0.099). Therefore, use of laboratory media in this and previous studies exaggerated adult pre-mating isolation and time to copulation in comparison to natural breeding substrates. These experiments suggest that a change in host substrates by saprophagous insects (where chemical differences exist between hosts) may have subtle effects on mating behaviour in a manner which promotes low levels of sexual isolation as a by-product of their utilization of a particular substrate during larval development. ForD. mojavensis, these results suggest that over evolutionary time, radiation into a new environment (from Baja to the mainland) allowed utilization of new host plants that may have incidentally promoted the sexual isolation patterns that have been observed within this species.
Similar content being viewed by others
References
Baker, W.K. (1947) A study of the isolating mechanisms found inDrosophila arizonensis andD. mojavensis.Univ. Tex. Publ. 4720, 126–36.
Brazner, J.C. (1983) The influence of rearing environment on sexual isolation between populations ofDrosophila mojavensis: an alternative to the character displacement hypothesis. MS Thesis, Syracuse, NY, USA.
Carson, H.L. (1987) The contribution of sexual behavior to Darwinian fitness.Behav. Genet. 17, 597–611.
Carson, H.L. and Lande, R. (1984) Inheritance of a secondary sexual character inDrosophila silvestris.Proc. Natl Acad. Sci. USA 81, 6904–7.
Crow, J.F. (1942) Cross fertility and isolating mechanisms in theDrosophila mulleri group.Univ. Tex. Publ. 4228, 54–67.
Downing, R.J. (1985) The chemical basis for host plant selection inDrosophila mojavensis. MS Thesis, University of Denver, Denver, CO, USA.
Ehrman, L. and Wasserman, M. (1987) The significance of asymmetrical sexual isolation.Evol. Biol. 21, 1–20.
Etges, W.J. (1989) Evolution of developmental homeostasis inDrosophila mojavensis.Evol. Ecol. 3, 189–201.
Etges, W.J. (1990) Direction of life history evolution inDrosophila mojavensis. InEcological and Evolutionary Genetics of Drosophila, pp. 37–56. (J.S.F. Barker, W.T. Starmer and R.J. MacIntyre, eds), Plenum, New York, USA.
Etges, W.J. (1992) Premating isolation is determined by larval substrates in cactophilicDrosophila mojavensis.Evolution 46, 1945–50.
Etges, W.J. and Heed, W.B. (1987) Sensitivity to larval density in populations ofDrosophila mojavensis: influences of host plant variation on components of fitness.Oecologia 71, 375–81.
Etges, W.J. and Heed, W.B. (1992) Remating effects on the genetic structure of female life histories in populations ofDrosophila mojavensis.Heredity 68, 515–28.
Ewing, A.W. and Miyan, J.A. (1986) Sexual selection, sexual isolation, and the evolution of song in theDrosophila repleta group of species.Anim. Behav. 34, 421–9.
Fellows, D.P. and Heed, W.B. (1972) Factors affecting host plant selection in desert-adaptedDrosophila.Ecology 53, 850–8.
Fogleman, J.C. and Starmer, W.T. (1985) Analysis of community structure of yeasts associated with decaying stems of cactus. III.Stenocereus thurberi.Microbiol. Ecol. 11, 165–73.
Gibson, A.C. and Horak, K.E. (1978) Systematic anatomy and phylogeny of Mexican columnar cacti.Ann. Missouri. Bot. Gard. 65, 999–1057.
Giddings, L.V. and Templeton, A.R. (1983) Behavioral phylogenies and the direction of evolution.Science 220, 372–8.
Gilbert, D.G. and Starmer, W.T. (1985) Statistics of sexual isolation.Evolution 39, 1380–3.
Heed, W.B. (1978) Ecology and genetics of Sonoran DesertDrosophila. InProceedings in the Life Sciences, Ecological Genetics: The Interface (P.F. Brussard, ed.), pp. 109–26. Springer-Verlag, New York, USA.
Heed, W.B. (1982) The origin ofDrosophila in the Sonoran desert. InEcological Genetics and Evolution: The Cactus—Yeast—Drosophila Model System (J.S.F. Barker and W.T. Starmer, eds), pp. 65–80. Academic Press, Sydney, Australia.
Heed, W.B. (1989) Origin ofDrosophila of the Sonoran Desert revisited: in search of a founder event and the description of a new species in theeremophila complex. InGenetics, Speciation and the Founder Principle (L.V. Giddings, K.Y. Kaneshiro and W.W. Anderson, eds), pp. 253–78. Oxford University Press, New York, USA.
Heed, W.B. and Mangan, R.L. (1986) Community ecology of the Sonoran DesertDrosophila. InThe Genetics and Biology of Drosophila (M. Ashburner, H.L. Carson and J.N. Thompson Jr, eds), Vol. 3e, pp. 311–45. Academic Press, New York, USA.
Johnson, W.R. (1980) Chromosomal polymorphism in natural populations of the desert adapted species,Drosophila mojavensis. PhD Dissertation, University of Arizona, Tucson, AZ, USA.
Kaneshiro, K.Y. (1976) Ethological isolation and phylogeny of theplanitibia subgroup of HawaiianDrosophila.Evolution 30, 740–5.
Kaneshiro, K.Y. (1980) Sexual isolation, speciation, and the direction of evolution.Evolution 34, 437–44.
Kaneshiro, K.Y. and Giddings, L.V. (1987) The significance of asymmetrical sexual isolation and the formation of new species.Evol. Biol. 21, 29–44.
Kaul, D. and Parsons, P.A. (1965) The genotypic control of mating speed and duration of copulation inDrosophila pseudoobscura.Heredity 20, 381–92.
Kircher, H.W. (1982) Chemical composition of cacti and its relationship to Sonoran DesertDrosophila. InEcological Genetics and Evolution: The Cactus—Yeast—Drosophila Model System (J.S.F. Barker and W.T. Starmer, eds), pp. 143–58. Academic Press, Sydney, Australia.
Koepfer, H.R. (1987a) Selection for sexual isolation between geographic forms ofDrosophila mojavensis. I. Interactions between the selected forms.Evolution 41, 37–48.
Koepfer, H.R. (1987b) Selection for sexual isolation between geographic forms ofDrosophila mojavensis. II. Effects of selection on mating preference and propensity.Evolution 41, 1409–12.
Krebs, R.A. (1990) Courtship behaviour and control of reproductive isolation inDrosphila mojavensis: Genetic analysis of population hybrids.Behav. Genet. 20, 535–43.
Krebs, R.A. and Markow, T.A. (1989) Courtship behavior and control of reproductive isolation inDrosophila mojavensis.Evolution 43, 908–12.
Malagolowkin-Cohen, Ch., Simmons, A.S. and Levene, H. (1965) A study of sexual isolation between certain strains ofDrosophila paulistorum.Evolution 19, 95–103.
Markow, T.A. (1981) Courtship behavior and control of reproductive isolation betweenDrosophila mojavensis andDrosophila arizonensis.Evolution 35, 1022–6.
Markow, T.A. (1982) Mating systems of cactophilicDrosophila. InEcological Genetics and Evolution: The Cactus—Yeast—Drosophila Model System (J.S.F. Barker and W.T. Starmer, eds), pp. 273–87. Academic Press, Sydney, Australia.
Markow, T.A. (1991) Sexual isolation among populations ofDrosophila mojavensis.Evolution 45, 1525–9.
Markow, T.A. and Toolson, E.C. (1990) Temperature effects on epicuticular hydrocarbons and sexual isolation inDrosophila mojavensis. InEcological and Evolutionary Genetics of Drosophila (J.S.F. Barker, W.T. Starmer and R.J. MacIntyre, eds), pp. 315–31, Plenum, New York, USA.
Markow, T.A., Fogleman, J.C. and Heed, W.B. (1983) Reproductive isolation in Sonoran DesertDrosophila.Evolution 37, 649–52.
Mettler, L.E. (1957) Studies on experimental populations ofDrosophila arizonensis andDrosophila mojavensis.Univ. Tex. Publ. 5721, 157–81.
Ohta, A. (1978) Ethological isolation and phylogeny in thegrimshawi species complex of HawaiianDrosophila.Evolution 32, 485–92.
Paterson, H.E.H. (1978) More evidence against speciation by reinforcement.S. Afr. J. Sci. 74, 369–71.
Pielou, E.C. (1977)Mathematical Ecology. Wiley, NY, USA.
Robertson, F.W. (1960) The ecological genetics of growth inDrosophila. 3. Growth and competitive ability of strains selected on different diets.Genet. Res. 1,333–50.
Royes, W.V. and Robertson, F.W. (1964) The nutritional requirements and growth relations of different species ofDrosophila.J. Exp. Zool. 156, 105–36.
Ruiz, A. and Heed, W.B. (1988) Host plant specificity in the cactophilicDrosophila mulleri species complex.J. Anim. Ecol. 57, 237–49.
Sauer, J.R. and Williams, B.K. (1989) Generalized procedures for testing hypotheses about survival or recovery rates.J. Wildl. Manage. 53, 137–42.
Spiess, E.B. (1987) Discrimination among prospective mates inDrosophila. InKin Recognition in Animals (D.J.C. Fletcher and C.D. Michener, eds), pp. 75–121. John Wiley and Sons Ltd, New York, USA.
Spiess, E.B. and Spiess, L.D. (1967) Mating propensity, chromosomal polymorphism, and dependent conditions inDrosophila persimilis.Evolution 21, 672–8.
Stalker, H.D. (1942) Sexual isolation studies in the species complexDrosophila virilis.Genetics 27, 238–57.
Starmer, W.T. (1982) Associations and interactions among yeasts,Drosophila, and their habitats. InEcological Genetics and Evolution: The Cactus—Yeast—Drosophila Model System (J.S.F. Barker and W.T. Starmer, eds), pp. 159–74. Academic Press, Sydney, Australia.
Starmer, W.T. and Gilbert, D.G. (1982) A quick and reliable method for sterilizing eggs.Dros.Inf.Serv. 58, 170.
Toolson, E.C. and Kuper-Simbron, R. (1989) Laboratory evolution of epicuticulan hydrocarbon composition and cuticular permeability inDrosophila pseudoobscura: Effects of sexual dimorphism and thermal-acclimation ability.Evolution 43, 468–72.
Toolson, E.C., Markow, T.A., Jackson, L.L. and Howard, R.W. (1990) Epicuticular hydrocarbon composition of wild and laboratory-rearedDrosophila mojavensis Patterson and Crow (Diptera: Drosophilidae).Ann. Entomol. Soc. Am. 83 1165–76.
Wasserman, M. (1982) Evolution of therepleta group. InThe Genetics and Biology of Drosophila, (M. Ashburner, H.L. Carson and J.N. Thompson, Jr, eds), Vol. 3b, pp. 61–139. Academic Press, New York, USA.
Wasserman, M. and Koepfer, R.H. (1977) Character displacement for sexual isolation betweenDrosophila mojavensis andDrosophila arizonensis.Evolution 31, 812–23.
Wasserman, M. and Koepfer, R.H. (1980) Does asymmetrical mate preference show the direction of evolution?Evolution 34, 1116–26.
Wasserman, M. and Zweig, H. (1991) Sexual preference for females reared on cactus media byDrosophila pegasa males.Evolution 45, 433–5.
Watanabe, T.K. and Kawanishi, M. (1979) Mating preference and the direction of evolution inDrosophila.Science 205, 906–7.
Yule, G.U. (1912) On the methods of measuring association between two attributes.J.R. Stat. Soc. 75, 579–642.
Zouros, E. and D'Entremont, C.J. (1974) Sexual isolation among populations ofDrosophila mojavensis race B.Dros.Inf.Serv. 51, 112.
Zouros, E. and D'Entremont, C.J. (1980) Sexual isolation among populations ofDrosophila mojavensis: response to pressure from a related species.Evolution 34, 421–30.
Author information
Authors and Affiliations
Additional information
See Etges (1992) for the first paper in this series.
Rights and permissions
About this article
Cite this article
Brazner, J.C., Etges, W.J. Pre-mating isolation is determined by larval rearing substrates in cactophilicDrosophila mojavensis. II. Effects of larval substrates on time to copulation, mate choice and mating propensity. Evol Ecol 7, 605–624 (1993). https://doi.org/10.1007/BF01237824
Issue Date:
DOI: https://doi.org/10.1007/BF01237824