Summary
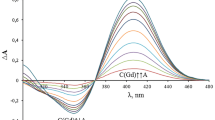
The absorption spectra (in the visible and ultraviolet) of the complexes formed in absolute methanol between Zr4+, V4+, Y3+ and Sb3+ with 3-hydroxyflavone, apigenin, hesperidin, naringenin, morin, kaempferol, quercetin, rutin and myricetin have been studied. Zr4+ and Sb3+ form complexes that are stable in acid medium. The stoichiometry of the vairous Zr4+-flavonoid complexes formed in methanol and methanol/HClO4 media has been determined by the molar ratio method. The preferred sites for complex formation of flavonoids with Zr4+ are postulated. Zr4+ forms chelates with the 3-hydroxy-4-keto and 5-hydroxy-4-keto systems simultaneously. Sb3+ forms complexes only with the 3,5-dihydroxy system in flavonoids. The results permit estimation of the relative order of chelating power of each type of binding site and are useful in elucidation of the structure of the flavonoids.
Zusammenfassung
Die Absorptions-Spektren (im sichtbaren und UV-Bereich) der in absolutem Methanol hergestellten Komplexe von Zr4+, V4+, Y3+ und Sb3+ mit 3-Hydroxyflavon, Apigenin, Hesperidin, Naringenin, Morin, Kaempferol, Quercetin, Rutin und Myricetin wurden untersucht. Zr4+ und Sb3+ bilden in saurem Milieu beständige Komplexe. Die Stöchiometrie der verschiedenen Zr4+-Flavonoid-Komplexe, die in Methanol bzw. Methanol/ HClO4 hergestellt wurden, wurde durch Bestimmung der Molarverhältnisse ermittelt. Die für die Komplexbildung bevorzugten Stellen (im Molekül) wurden angegeben. Zr4+ bildet Chelate mit der 3-Hydroxy-4-keto- und mit der 5-Hydroxy~4-ketogruppe. Sb3+ bildet solche Komplexe nur mit der 3,5-Dihydroxy-4-keto-Gruppe in Flavonoiden. Die Untersuchungsergebnisse ermöglichen die Abschätzung der Komplexbildungsfähigkeit der verschiedenen Gruppierungen und sind für die Strukturaufklärung von Nutzen.
Similar content being viewed by others
References
L. E. Dowd, Analyt. Chemistry31, 1184 (1959).
G. P. Kaushal, B. S. Sekhon, and I. S. Bhatia, Mikrochim. Acta [Wien]1979 I, 365.
I. S. Bhatia, J. Singh, and K. L. Bajaj, Mikrochim. Acta [Wien]1974, 909.
L. Jurd and T. A. Geissman, J. Org. Chem.21, 1395 (1956).
T. A. Geissman, in: The Chemistry of Flavonoid Compounds, Oxford: Pergamon. 1962.
K. R. Markham and T. J. Mabry, in: The Flavonoids J. B. Harborne, T. J. Mabry, and H. Mabry (eds.), Chapter 2, London: Chapman & Hall. 1975.
L. J. Porter and K. R. Markham, J. Chem. Soc. (C)1970, 344, 1309.
J. A. Mears and T. J. Mabry, Phytochem.11, 411 (1972).
M. Katyal and S. Prakash, Talanta24, 367 (1977).
W. C. Vosburgh and G. R. Cooper, J. Amer. Chem. Soc.63, 437 (1941).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sekhon, B.S., Kaushal, G.P. & Bhatia, I.S. Use of zirconium(IV) and antimony(III) for structural investigation of flavonoids. Mikrochim Acta 80, 421–427 (1983). https://doi.org/10.1007/BF01202020
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01202020