Summary
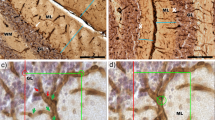
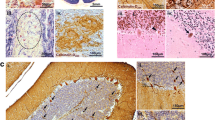
The hyperspiny Purkinje cell (hpc) is a murine, autosomal recessive mutation affecting cerebellar Purkinje cells. Axonal abnormalities in these neurons have been revealed by selective silver impregnation, specific immunohistochemical staining and electron microscopy. The main pathological feature consists of a massive axonal degeneration in the terminal domains of the Purkinje cell projection. This process starts approximately ten days postnatally, simultaneously with the onset of cerebellar symptoms, and evolves very rapidly. By 21 days, the vast majority of the terminal arbors have degenerated, resulting in an almost complete disruption of the corticonuclear projection. Axonal degeneration, although proceeding in a dying-back fashion, only provokes retrograde death in a small percentage of Purkinje cells (less than 15%).
Purkinje cells exhibit other signs of axonal damage and axonal reaction: (a) Almost all of them bear gigantic varicosities (spheroids or torpedoes) along their transit through the granular layer. (b) In a small percentage of cases, a dendritic segment is inserted between the axon hillock and the initial segment (meganeurite). These ectopic dendrites receive a normal contingent of synaptic inputs, and are transient structures observed in four- to six-week-old mice, (c) The infra- and supraganglionic plexuses, formed by recurrent collaterals of Purkinje cell axons, have increased density and terminal domains, (d) In mice aged over 50 days, many Purkinje cells have developed ‘arciform’ axons, which is evidence of a compensatory reaction.
The definite axonal pathology ofhpc Purkinje cells confers to this mutation its own specificity, which differs from all other known mutations primarily affecting this neuronal population. Therefore, thehpc mutation offers a valuable tool to analyse some of the genetic factors involved in the differentiation and maintenance of cerebellar Purkinje cells.
Similar content being viewed by others
References
Angaut, P. &Sotelo, C. (1973) The fine structure of the cerebellar central nuclei in the cat. II. Synaptic organization.Experimental Brain Research 16, 431–54.
Benedetti, F., Montarolo, P. G. &Rabacchi, S. (1984) Inferior olive lesion induces long-lasting functional modifications in the Purkinje cells.Experimental Brain Research 55, 368–71.
Caddy, K. W. T. &Biscoe, T. J. (1979) Structural and quantitative studies on the normal C3H and Lurcher mutant mouse.Philosophical Transactions of the Royal Society 287B, 167–201.
Chan-Palay, V. (1973) Neuronal plasticity in the cerebellar cortex and lateral nucleus.Zeitschrift für Anatomie und Entwicklungs-Geschichte 142, 23–35.
Chan-Palay, V. (1977)Cerebellar Dentate Nucleus. Organization, Cytology and Transmitters, Berlin: Springer-Verlag.
Colin, F., Manil, J. &Desclin, J. C. (1980) The olivocerebellar system. I. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibres.Brain Research 187, 3–27.
Desclin, J. C. &Colin, F. (1980) The olivocerebellar system: II. Some ultrastructural correlates of inferior olive destruction in the rat.Brain Research 187, 29–46.
Gentschev, T. &Sotelo, C. (1973) Degenerative patterns in the ventral cochlear nucleus of the rat after primary deafferentation. An ultrastructural study.Brain Research 62, 37–60.
Grillo, M. A. (1970) Cytoplasmic inclusions resembling nucleoli in sympathetic neurons of adult rats.Journal of Cell Biology 45, 100–17.
Guastavino, J. M., Sotelo, C. &Damez-Kinselle, I. (1990) Hot-foot murine mutation: Behavioral effects and neuroanatomical alterations.Brain Research (in press).
Guénet, J. L., Sotelo, C. &Mariani, J. (1983) Hyperspiny Purkinje cell, a new neurological mutation in the mouse.Journal of Heredity 74, 105–8.
Ito, M., Nisimaru, N. &Shibuki, K. (1979) Destruction of the inferior olive induces rapid depression in synaptic action of cerebellar Purkinje cells.Nature 277, 568–9.
Jande, S. S., Maler, L. &Lawson, D. E. M. (1981) Immunohistochemical mapping of vitamin D-dependent calcium binding protein in brain.Nature 294, 765–7.
Lampert, P. W. (1967) A comparative electron microscopic study of reactive, degenerating and dystrophic axons.Journal of Neuropathology and Experimental Neurology 26, 345–68.
Landis, S. C. (1973) Ultrastructural changes in the mitochondria of cerebellar Purkinje cells of nervous mutant mice.Journal of Cell Biology 57, 782–97.
Landis, S. C. &Mullen, R. J. (1978) The development and degeneration of Purkinje cells in pcd mutant mice.Journal of Comparative Neurology 177, 125–44.
Montarolo, P. G., Palestini, M. &Strata, P. (1982) The inhibitory effect of the olivocerebellar input on the Purkinje cell in the rat.Journal of Physiology 332, 187–202.
Oertel, W. H., Schmechel, D. E., Mugnaini, E., Tappaz, M. L. &Kopin, I. J. (1981) Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum.Neuroscience 6, 2715–35.
Palay, S. L. &Chan-Palay, V. (1974)Cerebellar Cortex, Cytology and Organization. Berlin: Springer-Verlag.
Palay, S. L., Sotelo, C., Peters, A. &Orkand, P. M. (1968) The axon hillock and the initial segment.Journal of Cell Biology 38, 193–201.
Prineas, J. (1969) The pathogenesis of dying-back polyneuropathies. Part II. An ultrastructural study of experimental acrylamide intoxication in the cat.Journal of Neuropathology and Experimental Neurology 28, 598–621.
Purpura, D. P. (1978) Ectopic dendritic growth in mature pyramidal neurons in human ganglioside storage disease.Nature 276, 520–1.
Purpura, D. P. (1979) Pathobiology of cortical neurons in metabolic and unclassified amentias. InCongenital and Acquired Cognitive Disorders (edited byKatzman, R.) pp. 43–48. New York: Raven.
Purpura, D. P. &Baker, H. J. (1977) Neurite induction in mature cortical neurons in feline GM1-ganglioside storage disease.Nature 266, 553–4.
Purpura, D. P. &Suzuki, K. (1976) Distortion of neuronal geometry and formation of aberrant synapses in neuronal storage disease.Brain Research 116, 1–21.
Purpura, D. P., Pappas, G. D. &Baker, H. J. (1978) Fine structure of meganeurites and secondary growth processes in feline GM1-gangliosidosis.Brain Research 143, 1–12.
Ramony Cajal, S. (1959)Degeneration and Regeneration of the Nervous System Vol. II. New York: Hafner.
Ramony Cajal, S. &De Castro, F. (1972)Elementos de Técnica Micrografica del Sistema Nerviosa. Madrid: Salvat.
Rossi, F., Cantino, D. &Strata, P. (1987) Morphology of Purkinje cell axon terminals in intracerebellar nuclei following inferior olive lesion.Neuroscience 22, 99–112.
Sotelo, C. (1978) Purkinje cell ontogeny: Formation and maintenance of spines.Progress in Brain Research 48, 149–68.
Sotelo, C. &Guénet, J. L. (1983) Nodding, a new mutant of the mouse with cerebellar abnormalities.Neuroscience Letters Supplement 14, S353.
Sotelo, C. &Palay, S. L. (1968) The fine structure of the lateral vestibular nucleus in the rat. I Neurons and neuroglial cells.Journal of Cell Biology 36, 151–79.
Sotelo, C. &Palay, S. L. (1971) Altered axons and axon terminals in the lateral vestibular nucleus of the rat.Laboratory Investigation 25, 653–71.
Sotelo, C. &Triller; A. (1979) Fate of presynaptic afferents to Purkinje cells in the adult nervous mutant mouse: A model to study presynaptic stabilization.Brain Research 175, 11–36.
Sternberger, L. A., Hardy, P. H. Jr, Cuculis, J. J. &Meyer, H. G. (1970) The unlabelled antibody method of immunohistochemistry. Preparation and properties of soluble antigen complex and its use in identification of spirochetes.Journal of Histochemistry and Cytochemistry 18, 315–33.
Walkley, S. U. (1982) Feline models of the gangliosidoses: Research applications for investigating the pathogenesis of lysosomal storage disease.Einstein Quarterly Journal of Biology and Medicine 1, 12–21.
Walkley, S. U., Blakemore, W. F. &Purpura, D. P. (1981) Alterations in neuron morphology in feline mannosidosis.Acta Neuropathologica 53, 75–9.
Walkley, S. U., Siegel, D. A. &Wurzelmann, S. (1988) Ectopic dendritogenesis and associated synapse formation in swainsonine-induced neuronal storage disease.Journal of Neuroscience 8, 445–57.
Wassef, M., Sotelo, C., Cholley, B., Brehier, A. &Thomasset, M. (1987) Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells.Developmental Biology 124, 379–89.
Williams, R. S., Gott, I. T., Ferrante, R. J. &Caviness, V. S. (1977) The cellular pathology of neuronal ceroidlipofuscinosis.Archives of Neurology 34, 298–305.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sotelo, C. Axonal abnormalities in cerebellar Purkinje cells of the ‘hyperspiny Purkinje cell’ mutant mouse. J Neurocytol 19, 737–755 (1990). https://doi.org/10.1007/BF01188042
Issue Date:
DOI: https://doi.org/10.1007/BF01188042