Abstract
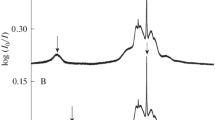
The structure I clathrate hydrate of carbon monoxide has been studied using dielectric measurements and13C NMR spectroscopy. Broad, weak dielectric absorption curves with maxima at 2.2–3.8 K yieldE a = 0.14 kJ mol−1 for the average Arrhenius activation energy associated with the reorientation of the low polarity guest. Except for H2S this represents the fastest reorienting polar guest known among the clathrate hydrates. The low temperature dielectric absorption curves can best be fitted with a Cole-Davidson asymmetric distribution of relaxation times and activation energies (withθ = 0.06 at 4 × 106 Hz), which at 107 Hz has been resolved into a double symmetric distribution of discrete relaxation times for CO in the small and large cages. The cross-polarization magic angle spinning13C NMR spectra indicate identical chemical shifts for CO in the small and large cages, in contrast to other hydrates. The static spectra show that the CO molecules undergo anisotropic reorientation in the large cages and that there is still considerable mobility at 77 K. One possible model for the anisotropic motion has the CO rapidly moving among sites over each of the 14 faces of the cage with the CO axis orientated towards the cage centre. The cage occupancy ratio at 220 K,θ s/θ L = 1.11, indicates slightly greater preference of CO for the small cage.
Similar content being viewed by others
References
D. W. Davidson, M. A. Desando, S. R. Gough, Y. P. Handa, C. I. Ratcliffe, J. A. Ripmeester, and J. S. Tse:Nature 328, 418 (1987).
D. W. Davidson, Y. P. Handa, C. I. Ratcliffe, J. S. Tse, and B. M. Powell:Nature 311, 142 (1984).
J. S. Tse, Y. P. Handa, C. I. Ratcliffe, and B. M. Powell:J. Incl. Phenom. 4, 235 (1986).
D. W. Davidson, Y. P. Handa, and J. A. Ripmeester;J. Phys. Chem. 90, 6549 (1986).
D. W. Davidson, Y. P. Handa, C. I. Ratcliffe, J. A. Ripmeester, J. S. Tse, J. R. Dahn, F. Lee, and L. D. Calvert:Mol. Cryst. Liq. Cryst. 141, 141 (1986).
A. Bar-Nun, G. Herman, D. Laufer, and M. L. Rappaport:Icarus 63, 317 (1985).
G. J. Consolmagno:J. Phys. Chem. 87, 4204 (1983).
J. Klinger:J. Phys. Chem. 87, 4209 (1983).
A. H. Delsemme:J. Phys. Chem. 87, 4214 (1983).
S. Wyckoff:J. Phys. Chem. 87, 4234 (1983).
J. M. Greenberg, C.E.P.M. van de Bult, and L. J. Allamandola:J. Phys. Chem. 87, 4243 (1983).
D. F. Strobel:Intl. Rev. Phys. Chem. 3, 145 (1983).
S. L. Miller, inIces in the Solar System, eds. J. Klinger, D. Benest, A. Dollfus, and R. Smoluchowski, Reidel, Dordrecht, 1985, p. 59.
J. I. Lunine and D. J. Stevenson:Astrophys. J. Suppl. Ser. 58, 493 (1985).
D. W. Davidson, C. I. Ratcliffe, and J. A. Ripmeester:J. Incl. Phenom. 2, 239 (1984).
C. I. Ratcliffe and J. A. Ripmeester:J. Phys. Chem. 90, 1259 (1986).
M. J. Collins, D. W. Davidson, C. I. Ratcliffe, and J. A. Ripmeester: inDynamics of Molecular Crystals, ed. J. Lascombe, Elsevier Science Publishers B.V., Amsterdam, 1987, p. 497.
S. R. Gough:J. Phys. E. Sci. Instrum. 15, 530 (1982).
A. Pines, M. C. Gibby, and J. S. Waugh:J. Chem. Phys. 59, 569 (1973).
A. L. McClellan:Tables of Experimental Dipole Moments, vol. 2, Rahara Enterprises, El Cerrito, California, 1974.
B. E. Read and G. Williams:Trans. Faraday Soc. 57, 1979 (1961).
D. W. Davidson and J. A. Ripmeester: inInclusion Compounds, eds. J. L. Atwood, J. E. D. Davies, and D. D. MacNicol, Academic Press, London, vol. 3, 1984, Ch. 3.
K. S. Cole and R. H. Cole:J. Chem. Phys. 9, 314 (1949).
R. M. Fuoss and J. G. Kirkwood:J. Am. Chem. Soc. 63, 385 (1941).
D. W. Davidson and R. H. Cole:J. Chem. Phys. 18, 1417 (1951).
S. Liu and M. S. Conradi:Solid State Commun. 49, 177 (1984).
K. R. Nary, P. L. Kuhns, and M. S. Conradi:Phys. Rev. B 26, 3370 (1982).
N. R. Grey and L. A. K. Staveley:Mol. Phys. 7, 83 (1983).
S. R. Gough, S. K. Garg, J. A. Ripmeester, and D. W. Davidson:J. Phys. Chem. 81, 2158 (1977).
S. R. Gough, R. E. Hawkins, B. Morris, and D. W. Davidson:J. Phys. Chem. 77, 2969 (1973).
A. Budd:Physik Z. 39, 706 (1938).
K. Bergman, D. M. Roberti, and C. P. Smyth:J. Phys. Chem. 5, 665 (1960).
J. H. Van Vleck:The Theory of Electric and Magnetic Susceptibilities, Oxford Press, New York, 1948.
L. Onsager:J. Am. Chem. Soc. 58, 1486 (1936).
D. A. Ackerman, D. Moy, R. C. Potter, and A. C. Anderson:Phys. Rev. B 23, 3886 (1981).
J. A. Ripmeester and C. I. Ratcliffe:J. Phys. Chem. 92, 337 (1988).
M. Mehring: inHigh Resolution NMR Spectroscopy in Solids, NMR Basic Principles and Progress, Eds. P. Diehl, E. Fluck, and R. Kosfeld, Springer-Verlag, New York, 1976, vol. 2, p 21.
A. J. Beeler, A. M. Orendt, D. M. Grant, P. W. Cutts, J. Michl, K. W. Zilm, J. W. Downing, J. C. Facelli, M. S. Schindler, and W. Kutzelnigg:J. Am. Chem. Soc. 106, 7672 (1984).
A. A. V. Gibson, T. A. Scott, and E. Fukushima:J. Magn. Reson. 27, 29 (1977).
J. S. Tse: National Research Council Canada, personal communication.
Tables of Interatomic Distances, Chemical Society Special Publication No. 11, London, 1958, M117.
F. A. Cotton and G. Wilkinson:Advanced Inorganic Chemistry, 2nd Edition, Interscience, New York, 1966, p. 115.
D. W. Davidson: inWater, a Comprehensive Treatise, ed. F. Franks, Plenum, New York, 1973, vol. 2, p. 130.
R. J. Wittebort, E. T. Olejniczak, and R. G. Griffin:J. Chem. Phys. 84, 5411 (1987).
C. I. Ratcliffe:J. Phys. Chem. 91, 6464 (1987).
G. A. Jeffrey: inInclusion Compounds, eds. J. L. Atwood, J. E. D. Davies, and D. D. MacNicol, Academic Press, London, Vol. 1, 1984, Ch. 5.
Author information
Authors and Affiliations
Additional information
Dedicated to Dr D. W. Davidson in honor of his great contributions to the sciences of inclusion phenomena.
Rights and permissions
About this article
Cite this article
Desando, M.A., Handa, Y.P., Hawkins, R.E. et al. Dielectric and13C NMR studies of the carbon monoxide clathrate hydrate. J Incl Phenom Macrocycl Chem 8, 3–16 (1990). https://doi.org/10.1007/BF01131283
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01131283