Summary
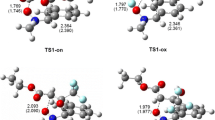
The global potential energy surface, determined in the first paper [1] for the groundstate ring opening of cyclopropylidene to allene, is complemented by accurate calculations of its key regions. The basis set is extended and polarization functions are included. The full configuration space of four electrons in four reactive orbitals is enlarged to the full configuration space of eight electrons in eight active orbitals by including correlations in the unbroken, but stretched CC sigma bonds. The effect of further single and double excitations is examined. The geometries and relative energies of the critical regions are found to change only little except for the ring-opening energy barrier which is lowered to about 7 kcal/mol, in good agreement with the experimental estimate of about 6 kcal/mol. Furthermore, the bifurcation is shown to occurafter the transition state, in the neighborhood of a conical intersection on the steepest descent path from the ring-opening transition state to the allene isomerization transition state. The steepest descent paths and the conical intersection are documented in detail. The cogwheel-like free internal rotation of the two methyl groups is confirmed by the accurate calculations. A similar richness of features is believed to exist on many potential energy surfaces governing chemical reactions.
Similar content being viewed by others
References
Valtazanos P, Elbert ST, Ruedenberg K (1991) Theor Chim Acta 78:287 (Paper 1)
Dykstra CE, Schaefer HF III (1980) in: Patai S (ed) The chemistry of ketenes, aldehydes and related compounds, Wiley Interscience, New York, p 1, and references therein
Ruedenberg K, Sundberg KR (1976) in: Galais JL, Linderberg J, Goscinski O, Ohrn Y (eds) Quantum science, structure and method, Plenum Press, New York
Dunning TH, Hay PJ (1971) in: Schaefer HF (ed) Methods of electronic structure theory, Plenum Press, New York, p 1
See Ref. [4]
Schmidt MW, Ruedenberg K (1979) J Chem Phys 71:3951
Raffenetti RC (1973) J Chem Phys 58:4452; Feller DF, Ruedenberg K (1979) Theor Chim Acta 52:231
Dupuis M, Spangler D, Wendoloski JJ (1980) Nat Resour Comput Chem Software Cat 1, Prog. No. QGO1 (GAMESS)
Elbert ST, Cheung LM, Ruedenberg K (1980) Nat Resour Comput Chem Software Cat 1, Prog. No. QMO1 (ALIS)
Edmiston C, Ruedenberg K (1963) Rev Mod Phys 35:457; England w, Salmon LS, Ruedenberg K (1971) Topics in Current Chemistry 23:31, and reference therein
Ruedenberg, Valtazanos P (1986) Theor Chim Acta 69:281
Herzberg G (1966) in: Molecular spectra and molecular structure III. Electronic spectra of polyatomic molecules, Van Nostrand Reinhold, New York, p 640
Warner P (private communication)
Xantheas S, Valtazanos P, Ruedenberg K (1991) Theor Chim Acta 78:327 (Paper 2)
Teller E (1937) J Phys Chem 41:109
Atchity G, Xantheas, S, Ruedenberg K (to be published)
Author information
Authors and Affiliations
Additional information
Operated for the U.S. Department of Energy by Iowa State University under Contract No. 7405-Eng-82. This work was supported by the Office of Basic Energy Sciences
Rights and permissions
About this article
Cite this article
Xantheas, S., Elbert, S.T. & Ruedenberg, K. The ring opening of cyclopropylidene to allene: key features of the accurate reaction surface. Theoret. Chim. Acta 78, 365–395 (1991). https://doi.org/10.1007/BF01112346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01112346