Abstract
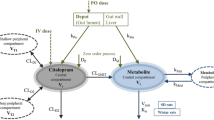
A prodrug of methyldopa, the pivaloyloxyethyl (POE) ester, was administered orally to healthy human volunteers at doses equivalent to 500 and 1000 mg of methyldopa and was compared to oral and intravenous doses of methyldopa. The time courses of availability of methyldopa to the general circulation were compared and contrasted with the model-independent estimates of total systemic availability. The POE ester of methyldopa is completely hydrolyzed on the first pass; delivery of methyldopa to the general circulation was faster, more uniform, and more extensive compared to orally administered methyldopa. The systemic availability of methyldopa averaged 64% of the dose with a coefficient of variation (CV) of 15% for the prodrug treatments compared to 27% of the dose with a CV of 63% for methyldopa. First-pass metabolism of drug to the mono-O-sulfate conjugate of methyldopa was lower for the POE ester than for methyldopa.
Similar content being viewed by others
References
K. C. Kwan, E. L. Foltz, G. O. Breault, J. E. Baer, and J. A. Totaro. Pharmacokinetics of methyldopa in man.J. Pharmacol. Exp. Ther. 198:264–277 (1976).
Ø. Stenbaek, E. Myhre, H. E. Rugstad, E. Arnold, and T. Hansen. Pharmacokinetics of methyldopa in healthy man.Eur. J. Clin. Pharmacol. 12:117–123 (1977).
J. J. Schrogie, R. O. Davies, S. S. Hwang, M. Hesney, G. O. Breault, K. C. Kwan, P. B. Huber, J. A. Feinburg, W. B. Abrams, and M. A. Zinney. Intrasubject variability in methyldopa bioavailability.Clin. Pharmacol. Ther. 25:248 (1979).
W. Y. W. Au, L. G. Dring, D. G. Grahame-Smith, P. Isaac, and R. T. Williams. The metabolism of14C-labelledα-methyldopa in normal and hypertensive human subjects.Biochem. J. 129:1–10 (1972).
W. S. Saari, M. B. Freedman, R. D. Hartman, S. W. King, A. W. Raab, W. C. Randall, E. L. Engelhardt, and R. Hirschmann. Synthesis and antihypertensive activity of some ester progenitors of methyldopa.J. Med. Chem. 21:746–753 (1978).
S. Vickers, C. A. Duncan, S. D. White, G. O. Breault, R. B. Royds, P. J. DeSchepper, and K. F. Tempero. Evaluation of succinimidoethyl and pivaloyloxyethyl esters as progenitors of methyldopa in man, rhesus monkey, dog, and rat.Drug Metab. Dispos. 6:640–646 (1978).
K. C. Yeh. Kinetic parameter estimation by numerical algorithms and multiple linear regression: theoretical.J. Pharm. Sci. 66:1688–1691 (1977).
K. C. Yeh and K. C. Kwan. Kinetic parameter estimation by numerical algorithms and multiple linear regression: application to pharmacokinetics.J. Pharm. Sci. 68:1120–1124 (1979).
M. R. Dobrinska and P. G. Welling. Pharmacokinetics of methsuximide and a major metabolite in dogs.J. Pharm. Sci. 66:688–692 (1977).
M. Rowland and G. Tucker. Symbols in pharmacokinetics.J. Pharmacokin. Biopharm. 8:497–507 (1980).
K. C. Kwan and A. E. Till. A novel method for bioavailability assessment.J. Pharm. Sci. 62:1494–1497 (1973).
J. C. K. Loo and S. Riegelman. New method for calculating the intrinsic absorption rate of drugs.J. Pharm. Sci. 57:918–928 (1968).
H. G. Boxenbaum and S. A. Kaplan. Potential source of error in absorption rate calculations.J. Pharmacokin. Biopharm. 3:257–264 (1975).
J. H. Zar.Biostatistical Analysis. Prentice-Hall, Englewood Cliffs, N.J., 1974, pp. 103–105.
C. P. Han. Testing the homogeneity of variances in a two-way classification.Biometrics 25:153–158 (1969).
C. T. Dollery and C. J. Bulpitt. Alpha-methyldopa in the treatment of hypertension: long-term experience InHypertension: Mechanisms and Management, G. Onesti, K. E. Kim, and J. H. Moyer (eds.), Grune & Stratton, New York, 1973, pp. 299–304.
R. P. Buhs, J. L. Beck, O. C. Speth, J. L. Smith, N. R. Trenner, P. J. Cannon, and J. H. Laragh. The metabolism of methyldopa in hypertensive human subjects.J. Pharmacol. Exp. Ther. 143:205–214 (1964).
A. Sjoerdsma, A. Vendsalu, and K. Engelman. Studies on the metabolism and mechanism of action of methyldopa.Circulation 28:492–502 (1963).
M. Marvola, P. Jokila, L. Niittyranta, M. Komulainen, and R. Hiltunen. Pharmacokinetics of methyldopa after oral administration in three different tablet formulations.Acta Pharm. Fenn. 89:109–114 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dobrinska, M.R., Kukovetz, W., Beubler, E. et al. Pharmacokinetics of the pivaloyloxyethyl (POE) ester of methyldopa, a new prodrug of methyldopa. Journal of Pharmacokinetics and Biopharmaceutics 10, 587–600 (1982). https://doi.org/10.1007/BF01062542
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01062542