Abstract
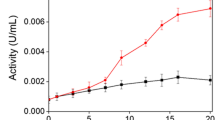
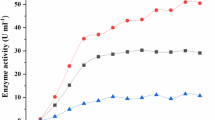
Laccases from the fungiRhizoctonia praticola andTrametes versicolor as well as horseradish peroxidase and tyrosinase were evaluated for their ability to polymerize phenolic contaminants. The removal of phenols through polymerization depended on the chemical structure and concentration of the substrate, pH of the reaction mixture, activity of the enzyme, length of incubation, and temperature. The enzymes retained their activity throughout a broad range of pH (pH 3.0 to 10) and temperature (5 to 55°C). The removal of halogenated phenols decreased with increasing number of chlorines and increasing molecular weight of the substituent. Laccases fromR. praticola andT. versicolor removed 2,4-dichlorophenol at initial concentrations of up to 1,600 mg/L. The amount of the substrate removed increased with increasing enzyme activity. The precipitates formed during polymerization of 2,4-dichlorophenol constituted a mixture of oligomers with average molecular weights of up to 800 for the fraction soluble in dioxane. Mass spectra revealed the loss of chlorine atoms during enzymatic polymerization. The release of chloride ions into solution during polymerization amounted to up to 20% of the chlorine initially associated with the 2,4-dichlorophenol molecule. Dechlorination contributes to the overall detoxification effect which results from enzymatic polymerization.
Similar content being viewed by others
References
Bollag JM, Sjoblad RD, Liu SY (1979) Characterization of an enzyme fromRhizoctonia praticola which polymerizes phenolic compounds. Can J Microbiol 25:229–233
Dordick JS, Marietta MA, Klibanov AM (1987) Polymerization of phenols catalyzed by peroxidase in nonaqueous media. Biotechnol Bioengineer 30:31–36
Hoff T, Liu SY, Bollag JM (1985) Transformation of halogen-, alkyl-, and alkoxy-substituted anilines by a laccase ofTrametes versicolor. Appl Environ Microbiol 49:1040–1045
Iwasaki I, Utsumi S, Hagino K, Ozawa T (1956) A new spectrometric method for the determination of small amounts of chloride using the mercuric thiocyanate method. Bull Chem Soc Japan 29:860–864
Klibanov AM, Alberti BN, Morris ED, Felshin LM (1980) Enzymatic removal of toxic phenols and anilines from waste waters. J Appl Biochem 2:414–421
Klibanov AM, Morris ED (1981) Horseradish peroxidase for the removal of carcinogenic aromatic amines from water. Enzyme Microb Technol 3:119–122
Klibanov AM, Tu TM, Scott KP (1983) Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science 221:259–261
Leonowicz A, Edgehill RU, Bollag JM (1984) The effect of pH on the transformation of syringic and vanillic acids by the laccases ofRhizoctonia praticola andTrametes versicolor. Arch Microbiol 137:89–96
Leonowicz A, Sarkar JM, Bollag JM (1988) Improvement in stability of an immobilized fungal laccase. Appl Microbiol Biotechnol 29:129–135
Liu SY, Minard RD, Bollag JM (1981) Coupling reactions of 2,4-dichlorophenol with various anilines. J Agric Food Chem 29:253–257
Maloney SW, Manem J, Mallevialle J, Fiessinger F (1986) Transformation of trace organic compounds in drinking water by enzymatic oxidative coupling. Environ Sci Technol 20:249–253
Minard RD, Liu SY, Bollag JM (1981) Oligomers and quinones from 2,4-dichlorophenol. J Agric Food Chem 29:250–253
Saunders BC, Holmes-Siedle AG, Stark BP (ed) (1964) Peroxidase. Butter-worths, Washington, DC
Sarkar JM, Malcolm RL, Bollag JM (1988) Enzymatic coupling of 2,4-dichlorophenol to stream fulvic acid in the presence of oxidoreductases. Soil Sci Soc Am J 52:688–694
Shuttleworth KL, Bollag JM (1986) Soluble and immobilized laccase as catalysts for the transformation of substituted phenols. Enzyme Microb Technol 8:171–177
Simmons KE, Minard RD, Bollag JM (1987) Oligomerization of 4-chloroaniline by oxidoreductases. Environ Sci Technol 21:999–1003
Subba-Rao RV, Rubin HE, Alexander M (1982) Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol 43:1139–1150
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dec, J., Bollag, J.M. Detoxification of substituted phenols by oxidoreductive enzymes through polymerization reactions. Arch. Environ. Contam. Toxicol. 19, 543–550 (1990). https://doi.org/10.1007/BF01059073
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01059073