Abstract
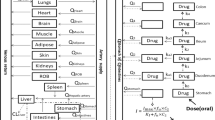
Sulfadimethoxine was administered intravenously and orally to five swine. More than 75% of the dose was excreted into urine as the acetyl metabolite with 4–6% excreted unchanged. Plasma and urine data were not consistent when a linear pharmacokinetic model was used to describe the data. Sulfadimethoxine has a high affinity for plasma protein, and the data were subsequently fitted to a nonlinear model, which included saturable protein binding. The choice of a nonlinear model was further supported by a minimum value for the Akaike information criteria. The protein binding constant obtained was 2.8× 104 M−1 and the total protein binding site concentration in plasma was 4.6×10−4 m. Both values are comparable with in vitrodata. This result suggests that the nonlinear model involving protein binding can be successfully applied to pharmacokinetic data. The apparent biological half-life of Sulfadimethoxine (free and bound) in plasma was 14 hr; however, the half-life of elimination of free drug was 1.25 hr. Following oral administration, all of the dose was absorbed with an apparent absorption half-life of 2.9 hr.
Similar content being viewed by others
References
D. W. A. Bourne. Drug residues in food animals.U.S. Pharmacist, 58–62 (1981).
W. G. Huber. The public health hazards associated with the nonmedical and animal health usage of antimicrobial drugs.Pure Appl. Chem. 35:377–388 (1972).
D. W. A. Bourne, R. F. Bevill, R. M. Sharma, R. P. Gural, and L. W. Dittert. Disposition of sulfonamides in food-producing animals: pharmacokinetics of sulfamethazine in lambs.Am. J. Vet. Res. 38:967–972 (1977).
G. D. Koritz, R. F. Bevill, D. W. A. Bourne, and L. W. Dittert. Disposition of sulfonamides in food-producing animals: pharmacokinetics of sulfathiazole in swine.Am. J. Vet. Res. 39:481–484 (1978).
R. F. Bevill, L. W. Dittert, and D. W. A. Bourne. Disposition of sulfonamides in food-producing animals. IV. Pharmacokinetics of sulfamethazine in cattle following administration of an intravenous dose and three oral dosage forms.J. Pharm. Sci. 66:619–623 (1977).
D. W. A. Bourne, M. Bialer, L. W. Dittert, M. Hayashi, G. Rudawsky, G. D. Koritz, and R. F. Bevill. Disposition of sulfadimethoxine in cattle: inclusion of protein binding factors in a pharmacokinetic model.J. Pharm. Sci. 70:1068–1072 (1981).
R. F. Bevill, R. M. Sharma, S. H. Meachum, S. C. Wozniak, D. W. A. Bourne, and L. W. Dittert. Disposition of sulfonamides in food-producing animals. Concentrations of sulfamethazine and its metabolites in plasma, urine, and tissues of lambs following intravenous administration.Am. J. Vet. Res. 38:973–977 (1977).
H. G. Boxenbaum and S. A. Kaplan. Potential source of error in absorption rate calculations.J. Pharmacokin. Biopharm. 3:257–264 (1975).
K. Yamaoka, T. Nakagawa, and T. Uno. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations.J. Pharmacokin. Biopharm. 6:165–175 (1978).
C. M. Metzler, G. L. Elfring, and A. J. McEwen.A Users Manual for NONLIN and Associated Programs. Research Biostatistics, Upjohn Company, Kalamazoo, Mich. 1974.
E. Genazzani and G. Pagnini. Binding capability of various sulfonamides to serum of different animal species.Am. J. Vet. Res. 24:1212–1216 (1963).
J. G. Wagner.Fundamentals of Clinical Pharmacokinetics. Drug Intelligence Publications, Hamilton, Ill., 1975.
P.-L. Hsu, J. K. H. Ma, H. W. Jun, and L. A. Luzzi. Structure relationship for binding of sulfonamides and penicillins to bovine serum albumin by fluorescence probe technique.J. Pharm. Sci. 63:27–31 (1974).
S. R. Walker. Influence of protein binding on the excretion of some sulfanilamidopyrimidines in man.J. Pharm. Pharmacol. 22:574–577 (1970).
W. Von Scholtan. Die hydrophobe bindung der pharmaka an human albumin and ribonucleinsaure.Arzneim. Forsch. 18:505–517 (1968).
M. S. Spector.Handbook of Biological Data, W. B. Saunders, Philadelphia, 1956.
Author information
Authors and Affiliations
Additional information
Supported in part by FDA contract 74–178. Presented at the APhA Academy of Pharmaceutical Sciences 25th National Meeting, Hollywood, Fla, November 12–16, 1978.
Rights and permissions
About this article
Cite this article
Bevill, R.F., Koritz, G.D., Rudawsky, G. et al. Disposition of sulfadimethoxine in swine: Inclusion of protein binding factors in a pharmacokinetic model. Journal of Pharmacokinetics and Biopharmaceutics 10, 539–550 (1982). https://doi.org/10.1007/BF01059036
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059036