Abstract
Purpose
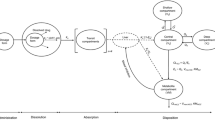
To establish an in vitro-in vivo correlation (IVIVC) model for Sporanox and SUBA-itraconazole formulations and to understand the impact of gastrointestinal (GI) pH and transit times on itraconazole dissolution and absorption.
Methods
IVIVC was developed based on fed/fasted pharmacokinetic data from randomized cross-over trials, in vitro dissolution studies, and prior information about typical and between subject variability of GI pH and transit times. Data were analysed using the population modelling approach as implemented in NONMEM.
Results
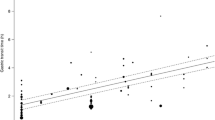
Dissolution kinetics were described using first order models. The in vivo pharmacokinetics of itraconazole was described with a 2-compartment model with 4-transit absorption compartments. Pharmacokinetic profiles for fasted itraconazole periods were described based on the in vitro dissolution model, in vivo disposition model, and the prior information on GI pH and transit times. The IVIVC model indicated that drug dissolution in the fed state required an additional pH-independent dissolution pathway. The IVIVC models were presented in a ‘Shiny’ application.
Conclusion
An IVIVC model was established and internally evaluated for the two itraconazole formulations. The IVIVC model provides more insight into the observed variability of itraconazole pharmacokinetics and indicated that GI pH and transit times influence in vivo dissolution and exposure.








Similar content being viewed by others
Abbreviations
- AIC:
-
Akaike information criteria
- AUC:
-
Area under the concentration-time curve
- BCS:
-
Biopharmaceutics classification system
- Cmax :
-
Maximum concentration
- GI:
-
Gastrointestinal
- GITT:
-
Gastrointestinal transit time
- HPLC:
-
High performance liquid chromatography
- IVIVC:
-
In vitro-in vivo correlation
- MOFV:
-
Minimum objective function value
- MTIME:
-
Model event time
- NONMEM:
-
Non-linear mixed effect modelling
- VPC:
-
Visual predictive check
References
Boogaerts M, Maertens J. Clinical experience with itraconazole in systemic fungal infections. Drugs. 2001;61(1):39–47.
De Beule K, Van Gestel J. Pharmacology of itraconazole. Drugs. 2001;61(1):27–37.
Peeters J, Neeskens P, Tollenaere JP, Van Remoortere P, Brewster ME. Characterization of the interaction of 2‐hydroxypropyl‐β‐cyclodextrin with itraconazole at pH 2, 4, and 7. J Pharm Sci. 2002;91(6):1414–22.
Poirier J-M, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet. 1998;35(6):461–73.
Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modelling of itraconazole and hydroxyl-itraconazole for oral SUBA-itraconazole and Sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015.
Yun H-y, Baek MS, Park IS, Choi BK, Kwon K-i. Comparative analysis of the effects of rice and bread meals on bioavailability of itraconazole using NONMEM in healthy volunteers. Eur J Clin Pharmacol. 2006;62(12):1033–9.
Jaruratanasirikul S, Kleepkaew A. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. Eur J Clin Pharmacol. 1997;52(3):235–7.
Smith D, Velde V, Woestenborghs R, Gazzard B. The pharmacokinetics of oral itraconazole in AIDS patients. J Pharm Pharmacol. 1992;44(7):618–9.
Janssen Pharmaceuticals Inc. Sporanox (itraconazole) capsules. Janssen Pharmaceuticals Inc, Beers, Belgium.
Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user’s guides, part V. (1989–2009). Ellicott City: Icon Development Solutions; 2009.
R Core Team. R: a language and environment for statistical computing Vienna, Austria R Foundation for Statistical Computing; 2014.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009.
Wickham H. plyr—the split-apply-combine strategy for data analysis. J Stat Softw. 2011;40(1):1–29.
Wickham H. Scales: Scale functions for graphics: CRAN.R-project.org; 2014.
Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994;22(5):431–45.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2, e38.
Wagner JG. Interpretation of percent dissolved‐time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci. 1969;58(10):1253–7.
Higuchi T. Mechanism of sustained‐action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52(12):1145–9.
Hixson A, Crowell J. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23(10):1160–8.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35.
Simonian HP, Vo L, Doma S, Fisher RS, Parkman HP. Regional postprandial differences in pH within the stomach and gastroesophageal junction. Dig Dis Sci. 2005;50(12):2276–85.
Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29(8):1035–41.
McCloy R, Greenberg G, Baron J. Duodenal pH in health and duodenal ulcer disease: effect of a meal, Coca-Cola, smoking, and cimetidine. Gut. 1984;25(4):386–92.
Ewe K, Press AG, Bollen S, Schuhn I. Gastric emptying of indigestible tablets in relation to composition and time of ingestion of meals studied by metal detector. Dig Dis Sci. 1991;36(2):146–52.
Davis S, Hardy J, Fara J. Transit of pharmaceutical dosage forms through the small intestine. Gut. 1986;27(8):886–92.
RStudio Inc. Shiny: web application framework for R. R package version 0.10.1. http://CRAN.R-project.org/package=shiny2015.
Abuhelwa AY, Foster DJ, Upton RN. ADVAN-style analytical solutions for common pharmacokinetic models. J Pharmacol Toxicol Methods. 2015;73:42–8.
Mooney K, Mintun M, Himmelstein K, Stella V. Dissolution kinetics of carboxylic acids II: effect of buffers. J Pharm Sci. 1981;70(1):22–32.
Lange D, Pavao JH, Wu J, Klausner M. Effect of a cola beverage on the bioavailability of itraconazole in the presence of H2 blockers. J Clin Pharmacol. 1997;37(6):535–40.
Clarke G, Newton J, Short M. Gastrointestinal transit of pellets of differing size and density. Int J Pharm. 1993;100(1):81–92.
Devereux J, Newton J, Short M. The influence of density on the gastrointestinal transit of pellets. J Pharm Pharmacol. 1990;42(7):500–1.
Abrahamsson B, Alpsten M, Jonsson UE, Lundberg P, Sandberg A, Sundgren M, et al. Gastro-intestinal transit of a multiple-unit formulation (metoprolol CR/ZOK) and a non-disintegrating tablet with the emphasis on colon. Int J Pharm. 1996;140(2):229–35.
Charman WN, Porter CJ, Mithani S, Dressman JB. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J Pharm Sci. 1997;86(3):269–82.
Food and Drug Administration. Guidance for industry: extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations. Center for Drug Evaluation and Research, Rockville. 1997.
Mayne Pharma International Pty Ltd. Lozanoc® (Itraconazole) 50 mg capsules: consumer medicine information. Available from: https://www.betterhealth.vic.gov.au/~/media/bhc/files/medicine%20guides%20library/11/cmi11099.pdf.
Banka S, Ryan K, Thomson W, Newman WG. Pernicious anemia—genetic insights. Autoimmun Rev. 2011;10(8):455–9.
Lake-Bakaar G, Quadros E, Beidas S, Elsakr M, Tom W, Wilson DE, et al. Gastric secretory failure in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1988;109(6):502–4.
Lu PJ, Hsu PI, Chen CH, Hsiao M, Chang WC, Tseng HH, et al. Gastric juice acidity in upper gastrointestinal diseases. World J Gastroenterol. 16(43):5496–501.
Weitschies W, Blume H, Mönnikes H. Magnetic marker monitoring: high resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur J Pharm Biopharm. 2010;74(1):93–101.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50:S41–67.
ACKNOWLEDGMENTS AND DISCLOSURES
All the pharmacokinetic studies used in the analysis were sponsored by Mayne Pharma International. S.M and D.H are employees at Mayne Pharma. R.U. has acted as a paid consultant for Mayne Pharma International. The Australian Centre for Pharmacometrics is an initiative of the Australian Government as part of the National Collaborative Research Infrastructure Strategy. A.Y.A is a PhD student receiving an Endeavour Scholarship funded by the Department of Education and Training of the Australian Government (Scholarship ID no. 4088).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 47 kb)
Rights and permissions
About this article
Cite this article
Abuhelwa, A.Y., Mudge, S., Hayes, D. et al. Population In Vitro-In Vivo Correlation Model Linking Gastrointestinal Transit Time, pH, and Pharmacokinetics: Itraconazole as a Model Drug. Pharm Res 33, 1782–1794 (2016). https://doi.org/10.1007/s11095-016-1917-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1917-1