Abstract
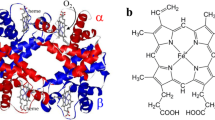
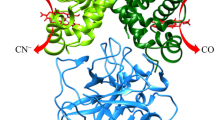
A specific intramolecular cross-link was introduced into bovine and human hemoglobin by reaction of the deoxyhemoglobin with the dialdehyde, bispyridoxal tetraphosphate (bisPL)P4, followed by reduction with NaBH4. The yield of cross-linked hemoglobin is 80% in both cases, using 1 mol of (bisPL)P4 per mol of Hb. The crosslink is confined to the β chains, where it connects the N-terminal residue (valine and methionine, respectively) to a lysine on the other β chain across the central cavity. The stereochemical requirements for the reaction were probed by using a rigid analogous cross-linking reagent, as well as with a mutant Hb, which has a shorter distance between the residues to which the cross-link is attached. Introduction of the cross-link into human and bovine Hb results in a five-fold and four-fold reduction in the oxygen affinity and a decrease in the Bohr Effect by 1/3 and 1/2, respectively. Oxygenation remains cooperative, albeit with a decreased Hill coefficient. The cross-linked hemoglobins are oxidized more rapidly to the ferric form, but their resistance to heat denaturation is increased. The stability of the link between the β chains and their hemes is 10 times greater in both cross-linked hemoglobins that in their native counterparts. The possible application of this chemical modification for the preparation of hemoglobin-based blood substitutes is discussed.
Similar content being viewed by others
References
Ames, B. N., and Dubin, D. T. (1960).J. Biol. Chem. 235, 769–775.
Barwick, R. C., Jones, R. T., Head, C. G., Shih, M. F.-C., Prchal, J. T., and Shih, D. T.-B. (1985).Proc. Natl. Acad. Sci. USA 82, 4602–4605.
Bellelli, A., Ippoliti, R., Brancaccio, A., Lendaro, E., and Brunori, M. (1990).J. Mol. Biol. 213, 571–574.
Benesch, R., Macduff, G., and Benesch, R. E. (1965a).Anal. Biochem. 11, 81–87.
Benesch, R. E., Benesch, R., and Macduff, G. (1965b).Proc. Natl. Acad. Sci. USA 54, 535–542.
Benesch, R. E., Benesch, R., Renthal, R. D., and Maeda, N. (1972).Biochemistry 11, 3576–3582.
Benesch, R., Benesch, R. E., Yung, S., and Edalji, R. (1975).Biochem. Biophys. Res. Commun. 63, 1123–1129.
Benesch, R. E., and Benesch, R. (1981).Methods in Enzymology 76, 147–159.
Benesch, R. E., and Kwong, S. (1988).Biochem. Biophys. Res. Commun. 156, 9–14.
Benesch, R. E., and Kwong, S. (1990).J. Biol. Chem. 265, 14,881–14,885.
Bucci, E., and Fronticelli, C. (1965).J. Biol. Chem. 240, PC 551.
Bunn, H. F., and Guidotti, G. (1972).J. Biol. Chem. 247, 2345–2350.
Chatterjee, R., Welty, E. V., Walder, R. Y., Pruitt, S. L., Rogers, P. H., Arnone, A., and Walder, J. A. (1986).J. Biol. Chem. 261, 9929–9937.
Clegg, J. B., Naughton, M. A., and Weatherall, D. J. (1965).Nature 207, 945–947.
Dixon, H. B. F., and McIntosh, R. (1967).Nature 213, 399–400.
Edelstein, S. J., Rehmar, M. J., Olson, J. S., and Gibson, Q. H. (1970).J. Biol. Chem. 245, 4372–4381.
Ip, S. H. J., Johnson, M. L., and Ackers, G. K. (1976).Biochemistry 15, 654–660.
Kavanaugh, M. P., Shih, D. T.-B., and Jones, R. T. (1988).Biochemistry 27, 1804–1808.
Keipert, P. E., Adeniran, A. J., Kwong, S., and Benesch, R. E. (1989).Transfusion 29, 768–773.
Michelson, A. M. (1964).Biochim. Biophys. Acta 91, 1–13.
Perutz, M. F., and Imai, K. (1980).J. Mol. Biol. 136, 183–191.
Pocker, A. (1973).J. Org. Chem. 38, 4295–5299.
Popp, F. D., and McEwen, W. E. (1958).Chem. Rev. 58, 321–401.
Severin, E. S., Gulyaev, N. N., Khurs, E. N., and Khomutov, R. M. (1969).Biochem. Biophys. Res. Commun. 35, 318–323.
Shimomura, S., and Fukui, T. (1978).Biochemistry 17, 5359–5367.
Tagaya, M., and Fukui, T. (1986).Biochemistry 25, 2958–2964.
Tagaya, M., Nakano, K., and Fukui, T. (1985).J. Biol. Chem. 260, 6670–6676.
Van Wazer, J. R. (1958).Phosphorus and Its Compounds, Interscience, New York.
Walder, J. A., Walder, R. Y., and Arnone, A. (1980).J. Mol. Biol. 141, 195–216.
White, F. L., and Olsen, K. W. (1987).Arch. Biochem. Biophys. 258, 51–57.
Yang, T., and Olsen, K. W. (1989).Biochem. Biophys. Res. Commun. 163, 733–738.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benesch, R.E., Kwong, S. Hemoglobin tetramers stabilized by a single intramolecular cross-link. J Protein Chem 10, 503–510 (1991). https://doi.org/10.1007/BF01025478
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01025478