Abstract
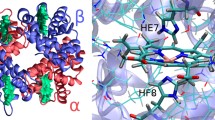
This mini-review, mainly based on our resonance Raman studies on the structural origin of cooperative O2 binding in human adult hemoglobin (HbA), aims to answering why HbA is a tetramer consisting of two α and two β subunits. Here, we focus on the Fe-His bond, the sole coordination bond connecting heme to a globin. The Fe-His stretching frequencies reflect the O2 affinity and also the magnitude of strain imposed through globin by inter-subunit interactions, which is the origin of cooperativity. Cooperativity was first explained by Monod, Wyman, and Changeux, referred to as the MWC theory, but later explained by the two tertiary states (TTS) theory. Here, we related the higher-order structures of globin observed mainly by vibrational spectroscopy to the MWC theory. It became clear from the recent spectroscopic studies, X-ray crystallographic analysis, and mutagenesis experiments that the Fe-His bonds exhibit different roles between the α and β subunits. The absence of the Fe-His bond in the α subunit in some mutant and artificial Hbs inhibits T to R quaternary structural change upon O2 binding. However, its absence from the β subunit in mutant and artificial Hbs simply enhances the O2 affinity of the α subunit. Accordingly, the inter-subunit interactions between α and β subunits are nonsymmetric but substantial for HbA to perform cooperative O2 binding.






Source: Nagai K, Kitagawa T, 1980


Similar content being viewed by others
References
Ackers GK, Perrella M, Holt JM, Denisov I, Huang Y (1997) Thermodynamics stability of the asymmetric doubly-ligated hemoglobin tetramer (α+CNβ+CN)(αβ): methodological and mechanistic issues. Biochemistry 36:10822–10829. https://doi.org/10.1021/bi971382x
Ackers GK (1998) Deciphering the molecular code of hemoglobin allostery. Adv Protein Chem 51:185–253. https://doi.org/10.1016/s0065-3233(08)60653-1
Ackers GK, Holt JM (2006) Asymmetric cooperativity in a symmetric tetramer: Human hemoglobin. J Biol Chem 281:1441–11443. https://doi.org/10.1074/jbc.R500019200
Adair GS (1925) The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J Biol Chem 63:529–545. https://doi.org/10.1016/S0021-9258(18)85018-9
Balakrishnan G, Case MA, Pevsner A, Zhao X, Tengroth C, McLendon GL, Spiro TG (2004) Time-resolved absorption and UV resonance Raman spectra reveal stepwise formation of T quaternary contacts in the allosteric pathway of hemoglobin. J Mol Biol 340:843–856. https://doi.org/10.1016/j.jmb.2004.05.012
Balakrishnan G, Zhao X, Podstawska E, Proniewicz LM, Kincaid JR, Spiro TG (2009) Subunit-selective interrogation of CO recombination in carbonmonoxy hemoglobin by isotope-edited time-resolved resonance Raman spectroscopy. Biochemistry 48:3120–3126. https://doi.org/10.1021/bi802190f
Barrick D, Ho NT, Simplaceanu V, Ho C (2001) Distal ligand reactivity and quaternary structure studies of proximally detached hemoglobins. Biochemistry 40:3780–3795. https://doi.org/10.1021/bi002165q
Bohr C, Hasselbalch K, Krogh A (1904) Über einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensaurespannung des Blutes auf dessen Sauerstoffbindung ubt. Skand Arch Physiol 16:402–412. https://doi.org/10.1111/j.1748-1716.1904.tb01382.x
Chang R, Thoman JW Jr (2014) Physical chemistry for the chemical and biological sciences. University Science Books
Richard V, Dodson GG, Mauguen Y (1993) Human deoxyhaemoglobin-2,3-diphosphoglycerate complex low-salt structure at 2.5 A resolution. J Mol Biol 233:270–274. https://doi.org/10.1006/jmbi.1993.1505
Hill AV (1913) The combination of haemoglobin with oxygen and with carbon monoxide. Biochem J 7:471–480. https://doi.org/10.1042/bj0070471
Harada I, Takeuchi H (1986) Raman and ultraviolet resonance Raman spectra of proteins and related compounds. In: Clark RJH, Hester RE (eds) Advances in spectroscopy, John Wiley & Sons, Chiester, 113–196
Henry ER, Bettati S, Hofrichter H, Eaton WA (2002) A tertiary two-state model for hemoglobin. Biophys Chem 98:149–164. https://doi.org/10.1016/s0301-4622(02)00091-1
Ho C (1992) Proton nuclear magnetic resonance studies on hemoglobin: cooperative interactions and partially ligated intermediates. Adv Protein Chem 43:153–312. https://doi.org/10.1016/S0065-3233(08)60555-0
Imai K (1982) Allosteric effects in haemoglobin. Cambridge University Press, Cambridge
Inubushi T, Ikeda-Saito M, Yonetani T (1983) Isotropically shifted NMR resonances for the proximal histidyl imidazole NH protons in cobalt hemoglobin and iron-cobalt hybrid hemoglobins. Binding of the proximal histidine toward porphyrin metal ion in the intermediate state of cooperative ligand binding. Biochemistry 22:2904–2907. https://doi.org/10.1021/bi00281a019
Johnson ME, Ho C (1974) Effects of ligands and organic phosphates on functional properties of human adult hemoglobin. Biochemistry 13:3653–3661. https://doi.org/10.1021/bi00715a005
Johnson ML, Ackers GK (1982) Thermodynamic analysis of human hemoglobin in terms of Perutz mechanism: extensions of the Szabo-Karplus model to include subunit assembly. Biochemistry 21:201–211. https://doi.org/10.1021/bi00531a001
Jones EM, Balakrishnan G, Spiro TG (2012) Heme reactivity in uncoupled from quaternary structure in gel-encapsulated hemoglobin: resonance Raman spectroscopic study. J Am Chem Soc 134:3461–3471. https://doi.org/10.1021/ja210126j
Jones EM, Monza E, Balakrishnan G, Blouin GC, Mak PJ, Zhu Q, Kincaid JR, Guallar V, Spiro TG (2014) Differential control of heme reactivity in alpha and beta subunits of hemoglobin: A combined Raman spectroscopic and computational study. J Am Chem Soc 136:10325–10339. https://doi.org/10.1021/ja503328a
Kitagawa T, Nagai K, Tsubaki M (1979) Assignment of the FeNε(His F8) stretching band in the resonance Raman spectra of deoxy myoglobin. FEBS Lett 104:376–379. https://doi.org/10.1016/0014-5793(79)80856-X
Kitagawa T, Nagai K (1982) Resonance Raman evidence for a stretched FeNε (His F8) bond in the T-structure deoxyhemoglobin. In: Ho C (ed) Hemoglobin and oxygen binding. Elsevier Biomedical, New York, pp 217–222
Kitagawa T (1988) The heme protein structure and the iron histidine stretching mode. In: Spiro TG (ed) Biological applications of Raman spectroscopy, vol 3. John Wiley & Sons, New York, pp 97–131
Koshland DE, Némethy G, Filmer D (1966) Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 5:365–385. https://doi.org/10.1021/bi00865a047
Kovalevsky AY, Chatake T, Shibayama Park SY, Ishikawa T, Mustyakimov M, Fisher Z, Langan P, Morimoto Y (2010) Direct determination of protonation states of histidine residues in a 2 Å neutron structure of deoxy-human normal adult hemoglobin and implications for the Bohr effect. J Mol Biol 398:276–291. https://doi.org/10.1016/j.jmb.2010.03.016
Makowski L, Bardhan J, Gore D, Lal J, Mandava S, Park S, Rodi DJ, Ho NT, Ho C, Fischetti RF (2011) WAXS studies of the structural diversity of hemoglobin in solution. J Mol Biol 408:909–921. https://doi.org/10.1016/j.jmb.2011.02.062
Marzocchi MP, Smulevich G (2003) Relationship between heme vinyl conformation and the protein matrix in peroxidases. J Raman Spectrosc 34:725–736. https://doi.org/10.1002/jrs.1037
Matsukawa S, Mawatari K, Yoneyama Y, Kitagawa T (1985) Correlation between the ironhistidine stretching frequencies and oxygen affinity of hemoglobins. A continuous strain model. J Am Chem Soc 107:1108–1113. https://doi.org/10.1021/ja00291a004
Monod J, Wyman J, Changeux P (1965) On the nature of allosteric transitions: A plausible model. J Mol Biol 12:88–118. https://doi.org/10.1016/s0022-2836(65)80285-6
Nagatomo S, Nagai M, Tsuneshige A, Yonetani T, Kitagawa T (1999) UV resonance Raman studies of α-nitrosyl hemoglobin derivatives: Relation between the α1-β2 subunit interface interactions and the Fe-histidine bonding of α heme. Biochemistry 38:9659–9666. https://doi.org/10.1021/bi990567w
Nagatomo S, Nagai M, Shibayama N, Kitagawa T (2002a) Differences in changes of the α1-β2 subunit contacts between ligand binding to the α and β subunits of hemoglobin A: UV resonance Raman analysis using Ni-Fe hybrid hemoglobin. Biochemistry 41:10010–10020. https://doi.org/10.1021/bi0200460
Nagatomo S, Jin Y, Nagai M, Hori H, Kitagawa T (2002b) Changes in the abnormal α-subunit upon CO-binding to the normal β-subunit of Hb M Boston: Resonance Raman, EPR, and CD study. Biophys Chem 98:217–232. https://doi.org/10.1016/S0301-4622(02)00103-5
Nagatomo S, Nagai M, Mizutani Y, Yonetani T, Kitagawa T (2005) Quaternary structures of intermediately ligated human hemoglobin A and influences from strong allosteric effectors: Resonance Raman investigation. Biophys J 89:1203–1213. https://doi.org/10.1529/biophysj.104.049775
Nagatomo S, Nagai M, Kitagawa T (2011a) A new way to understand quaternary strucature changes of hemoglobin upon ligand binding on the basis of UV-resonance Raman evaluation of inter-subunit interactions. J Am Chem Soc 133:10101–10110. https://doi.org/10.1021/ja111370f
Nagatomo S, Nagai M, Kitagawa T (2011b) Resonance Raman investigation of quaternary structure change in hemoglobin upon ligand binding. In: Nagai M (ed) Hemoglobin: Recent Developments and Topics, Research Signpost, Kerala, India, pp 37–61
Nagatomo S, Nagai Y, Aki Y, Sakurai H, Imai K, Mizusawa N, Ogura T, Kitagawa T, Nagai M (2015) An origin of cooperative oxygen binding of human adult Hemoglobin: different roles of the α and β subunits in the α2β2 tetramer. PLoS ONE 10:e0135080. https://doi.org/10.1371/journal.pone.0135080
Nagatomo S, Okumura M, Saito K, Ogura T, Kitagawa T, Nagai M (2017a) Interrelationship among the Fe-His bond strengths, oxygen affinities and intersubunit hydrogen-bonding changes upon ligand binding in β subunit of human hemoglobin; the alkaline Bohr effect. Biochemistry 56:1261–1273. https://doi.org/10.1021/acs.biochem.6b01118
Nagatomo S, Saito K, Yamamoto K, Ogura T, Kitagawa T, Nagai M (2017b) Heterogeneity between two α subunits of α2β2 human hemoglobin and O2 binding properties: Raman, 1H nuclear magnetic resonance, and terahertz spectra. Biochemistry 56:6125–6136. https://doi.org/10.1021/acs.biochem.7b00733
Nagatomo S, Kitagawa T, Nagai M (2021) Roles of Fe-His bonds in stability of hemoglobin: recognition of protein flexibility by Q Sepharose. Biophys J 120:1–12. https://doi.org/10.1016/j.bpj.2021.05.014
Nagai K, Kitagawa T (1980) Differences in Fe(II)-Nε(His-F8) stretching frequencies between deoxyhemoglobins in the two alternative quaternary structures. Proc Natl Acad Sci USA 77:2033–2037. https://doi.org/10.1073/pnas.77.4.2033
Nagai K, Kitagawa T, Morimoto H (1980) Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance Raman scattering. J Mol Biol 136:271–289. https://doi.org/10.1016/0022-2836(80)90374-5
Nagai M, Mizusawa N, Kitagawa T, Nagatomo S (2018) A role of heme side-chains of human hemoglobin in its function revealed by circular dichroism and resonance Raman spectroscopy. Biophys Rev 10:271–284. https://doi.org/10.1007/s12551-017-0364-5
Ohe M, Kajita A (1980) Changes in pKa values of individual histidine residues of human hemoglobin upon reaction with carbon monoxide. Biochemistry 19:4443–4450. https://doi.org/10.1021/bi00560a010
Paoli M, Dodson G, Liddington RC, Wilkinson AJ (1997) Tension in haemoglobin revealed by Fe-His(F8) bond rupture in the fully liganded T-state. J Mol Biol 271:161–167. https://doi.org/10.1006/jmbi.1997.1180
Park SY, Yokoyama T, Shibayama N, Shiro Y, Tame YJR (2006) 1.25 Å resolution crystal structures of human haemoglobin in the oxy, deoxy and carbonmonoxy forms. J Mol Biol 360:690–701. https://doi.org/10.1016/j.jmb.2006.05.036
Perutz MF, Rossmann MG, Cullis AF, Muirhead H, Will G, North AC (1960) Structure of haemoglobin: a three-dimensional Fourier synthesis at 5.5-A. Resolution, obtained by X-ray analysis. Nature 185:416–422. https://doi.org/10.1038/185416a0
Perutz MF (1970) Stereochemistry of cooperative effects in haemoglobin. Nature 228:726–739. https://doi.org/10.1038/228726a0
Perutz MF (1979) Regulation of oxygen affinity of hemoglobin: Influence of structure of the globin on the heme iron. Annu Rev Biochem 48:327–386. https://doi.org/10.1146/annurev.bi.48.070179.001551
Philo JS, Dreyer U, Lary JW (1996) Quaternary structure dynamics and carbon monoxide binding kinetics of hemoglobin valency hybrids. Biophys J 70:1949–1965. https://doi.org/10.1016/S0006-3495(96)79760-6
Sato A, Tai H, Nagatomo S, Imai K, Yamamoto Y (2011) Determination of oxygen binding properties of the individual subunits of intact human adult hemoglobin. Bull Chem Soc Jpn 84:1107–1111. https://doi.org/10.1246/bcsj.20110104
Shibayama N, Morimoto H, Miyazaki G (1986) Oxygen equilibrium study and light absorption spectra of Ni(II)-Fe(II) hybrid hemoglobins. J Mol Biol 192:323–329. https://doi.org/10.1016/0022-2836(86)90367-0
Shibayama N, Imai K, Morimoto H, Miyazaki G, Saigo S (1995) Oxygen equilibrium properties of Nickel(II)-Iron(II) hybrid hemoglobins cross-linked between 82β1 and 82β 2 lysyl residues by bis(3,5-dibromosalicyl)fumarate: determination of first two-step microscopic Adair constants for human hemoglobin. Biochemistry 34:4773–4780. https://doi.org/10.1021/bi00014a035
Shibayama N, Morimoto H, Saigo S (1997) Reexamination of the hyper thermodynamics stability of asymmetric cyanomet valency hybrid hemoglobin, (α+CN-β+CN-)(αβ): no preferentially populating asymmetric hybrid at equilibrium. Biochemistry 34:4375–4381. https://doi.org/10.1021/bi970009m
Shibayama N, Morimoto H, Saigo S (1998) Asymmetric cyanomet valency hybrid hemoglobin, (α+CN-β+CN-)(αβ): the issue of valency exchange. Biochemistry 37:6221–6228. https://doi.org/10.1021/bi980134d
Shibayama N, Saigo S (1999) Kinetics of the allosteric transition in hemoglobin within silicate sol-gels. J Am Chem Soc 121:444–445. https://doi.org/10.1021/ja983652k
Shibayama N, Saigo S (2001) Direct observation of two distinct affinity conformations in the T state human deoxyhemoglobin. FEBS Lett 492:50–53. https://doi.org/10.1016/S0014-5793(01)02225-6
Sugita Y, Yoneyama Y (1971) Oxygen equilibrium of hemoglobins containing unnatural Hemes. J Biol Chem 246:389–394. https://doi.org/10.1016/S0021-9258(18)62503-7
Szabo A, Karplus M (1972) A mathematical model for structure-function relations in hemoglobin. J Mol Biol 72:163–197. https://doi.org/10.1016/0022-2836(72)90077-0
Tyuma I, Shmizu K, Imai K (1971) Effect of 2,3-diphosphoglycerate on the cooperativity in oxygen binding of human adult hemoglobin. Biochem Biophys Res Commun 43:423–428. https://doi.org/10.1016/0006-291X(71)90770-4
Unzai S, Eich R, Shibayama N, Olson JS, Morimoto H (1998) Rate constants for O2 and CO binding to the α and β subunits within the R and T states of human hemoglobin. J Biol Chem 273:23150–23159. https://doi.org/10.1074/jbc.273.36.23150
Viappiani C, Bettati S, Bruno S, Ronda L, Abbruzzetti S, Mozzarelli A, Eaton WA (2004) New insights into allosteric mechanisms from trapping unstable protein conformations in silica gels. Proc Natl Acad Sci USA 101:14414–14419. https://doi.org/10.1073/pnas.0405987101
Viappiani C, Abbruzzetti S, Ronda L, Bettati S, Henry ER, Mozzarelli A, Eaton WA (2014) Experimental basis for a new allosteric model for multisubunit proteins. Proc Natl Acad Sci USA 111:12758–12763. https://doi.org/10.1073/pnas.1413566111
Yamada K, Ishikawa H, Mizuno M, Shibayama N, Mizutani Y (2013) Intersubunit communication via changes in hemoglobin quaternary structures revealed by time-resolved resonance Raman spectroscopy: direct observation of the Perutz mechanism. J Phys Chem B 117:12461–12468. https://doi.org/10.1021/jp407735t
Yamamoto H, Yonetani T (1974) Studies on modified hemoglobins: IV. Hybrid hemoglobins containing proto- and mesohemes and their oxygenation characteristics. J Biol Chem 249:7964–7968. https://doi.org/10.1016/S0021-9258(19)42059-0
Yonetani T, Tsuneshige A, Zhou Y, Chen X (1998) Electron paramagnetic resonance and oxygen binding studies of α-nitrosyl hemoglobin. J Biol Chem 273:20323–20333. https://doi.org/10.1074/jbc.273.32.20323
Yonetani T, Park SI, Tsuneshige A, Imai K, Kanaori K (2002) Global allostery model of hemoglobin. Modulation of O2 affinity, cooperativity, and Bohr effect by heterotropic allosteric effectors. J Biol Chem 277:34508–34520. https://doi.org/10.1074/jbc.M203135200
Yonetani T, Laberge M (2008) Protein dynamics explain the allosteric behaviors of hemoglobin. Biochim Biophys Acta 1784:1146–1158. https://doi.org/10.1016/j.bbapap.2008.04.025
Yonetani T, Kanaori K (2013) How does hemoglobin generate such diverse functionality of physiological relevance. Biochim Biophys Acta 1834:1873–1884. https://doi.org/10.1016/j.bbapap.2013.04.026
Yuan Y, Tam MF, Simplaceanu V, Ho C (2015) New look at hemoglobin allostery. Chem Rev 115:1702–1724. https://doi.org/10.1021/cr500495x
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology for Scientific Research (C) to S.N. (17K05606), (20K03877) and Scientific Research (B) to T.K. (24350086), and by a research grant from the Research Center for Micro-Nano Technology, Hosei University, to M.N.
Author information
Authors and Affiliations
Contributions
Shigenori Nagatomo had the idea of the manuscript. The first draft of the manuscript was written by Teizo Kitagawa. Shigenori Nagatomo and Masako Nagai commented on the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagatomo, S., Nagai, M. & Kitagawa, T. Structural origin of cooperativity in human hemoglobin: a view from different roles of α and β subunits in the α2β2 tetramer. Biophys Rev 14, 483–498 (2022). https://doi.org/10.1007/s12551-022-00945-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-022-00945-7