Abstract
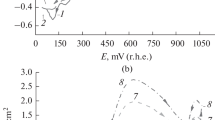
Voltammetry at Hg microelectrodes has been used to investigate the mechanism of the reduction of formaldehyde in conditions close to those employed for the electrosynthesis of ethylene glycol (that is, a very high concentration of formaldehyde in a neutral buffer at high temperature). It is shown that, even with a 40% formaldehyde solution, it is possible to record a well formed reduction wave, limiting current densities up to 30 A cm−2. The variations in half-wave potential, limiting current density and wave shape with formaldehyde concentration are reported and the influence of pH, temperature and electrolyte are considered. With increasing formaldehyde concentration, the transition from 2e− reduction to 1e− reduction is clearly observed. The results led to a re-examination of the influence of cathode material on the yield of ethylene glycol and it is confirmed that graphite is definitely the best choice.
Similar content being viewed by others
References
K. Vesely and R. Brdicka,Collect Czech. Chem. Commun. 12 (1947) 313.
R. Bieber and G. Trumpler,Helv. Chim. Acta. 30 (1947) 706.
R. Brdicka,Coll. Czech. Chem. Commun. 20 (1955) 387.
,Z. Elektrochem. 59 (1955) 787.
N. Landquist,Acta. Chem. Scand. 9 (1955) 867.
P. Valentli,Coll. Czech. Chem. Commun. 25 (1960) 853.
M. A. Loshkarev and A. J. Tschernikov,Russ. J. Phys. Chem. 27 (1953) 1778.
S. Clarke and J. A. Harrison,J. Electroanal. Chem. 36 (1972) 109.
A. Calusaru, I. Crisan and J. Kuta,46 (1973) 51.
J. M. Los, A. A. A. M. Brinkman and B. C. J. Wetsma,56 (1974) 187.
J. M. Los, L. F. Roeleveld and B. C. J. Wetsma,75 (1977) 819.
P. G. Russell, N. Kovacs, S. Srinivasan and M. Steinberg,J. Electrochem. Soc. 124 (1977) 1329.
A. P. Tomilov, E. L. Klyuev and V. D. Nechepornoi,Russ. J. Gen. Chem. 43 (1973) 2792.
11 (1975) 2010.
H. Watanabe and M. Saito,Toyo Soda Kenkyo, Hokoku 24 (1979) 93.
M. Saito and Y. Tokuyuma, German Pat. 3,018,844 (1980).
N. L. Weinberg, US Pat. 4,478,694 (1984).
N. L. Weinberg, M. Lipsztajn, D. J. Mazur, M. Reicher, H. R. Weinberg and E. P. Weinberg, in Recent Advances in Electroorganic Synthesis (edited by S. Torii) Elsevier (1987).
N. L. Weinberget al. to be published.
M. I. Montenegro and D. Pletcher,J. Electroanal. Chem. 248 (1988) 229.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Montenegro, M.I., Pletcher, D., Liolios, E.A. et al. A microelectrode study of the reduction of formaldehyde in neutral concentrated aqueous solutions. J Appl Electrochem 20, 54–59 (1990). https://doi.org/10.1007/BF01012471
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01012471