Abstract
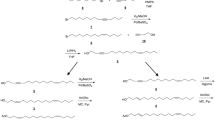
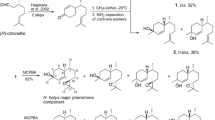
Among the pure stereoisomers of 5,9-dimethylheptadecane, a previously identified sex pheromone component ofLeucoptera scitella L., only theS,S isomer yielded trap captures in the field. The addition of the other stereoisomers had no effect on cathes. The addition of low percentages of racemic 5,9-dimethylhexadecane, a previously identified minor component in the sex pheromone, did not influence trap catches or alter behavior of males approaching an attractant source in the field.
Similar content being viewed by others
References
Arn, H., Rauscher, S., andSchmid, A. 1979. Sex attractant formulations and traps for the grape moth,Eupoecilia ambiguella Hb.Mitt. Schweiz. Entomal. Ges. 52:49–55.
Francke, W., Franke, S., Tóth, M., Szöcs, G., Guerin, P., andArn, H. 1987. Identification of 5,9-dimethylheptadecane as a sex pheromone of the mothLeucoptera scitella.Naturwissenschaften 74:143–144.
Francke et al. 1989
Helmchen, G., andWegner, G. 1985. Asymmetric synthesis of β-substituted alcanoic acids via highly stereoselective conjugate additions of organocopper compounds to chiral enoates.Tetrahedron Lett. 26:6051–6054.
Leikauf, U. 1988. Asymmetrische Synthesen mit Estern konkaver Bornanole: Stereoselektive Synthese des Sexualpheromons vonLeucoptera scitella; Untersuchungen zur asymmetrischen Mukaiyama-Reaktion. Dissertation, Universität Heidelberg, 1988.
Sato, R., Abe, N., Sonnet, P., Sugie, H., andTamaki, Y. 1985. Biological activity of (R)- and (S)-14-methyl-1-octadecene, as the chiral component of the sex pheromone of the peach leaf-miner moth,Lyonetia clerkella Linné (Lepidoptera: Lyonetiidae).Appl. Entomol. Zool. 20:411–415.
Schmierer, R., Grotemeier, G., Helmchen, G., andSelim, A. 1981. Functional groups at concave sites: Asymmetric alkylation of esters with very high stereoselectivity and reversal of configuration by change of solvent.Angew. Chem. Int. Ed. Engl. 20:207–208.
Sugie, H., Tamaki, Y., Sato, R., andKumakura, M. 1984. Sex pheromone of the peach leaf-miner moth,Lyonetia clerkella Linné: isolation and identification.Appl. Entomol. Zool. 19:323–330.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tóth, M., Helmchen, G., Leikauf, U. et al. Behavioral activity of optical isomers of 5,9-dimethylheptadecane, the sex pheromone ofLeucoptera scitella L. (Lepidoptera: Lyonetidae). J Chem Ecol 15, 1535–1543 (1989). https://doi.org/10.1007/BF01012381
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01012381