Abstract
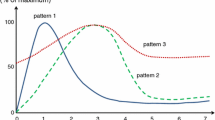
To clarify the relation between macrophage and myofibroblast involvement in various myocardial diseases, the authors investigated the kinetics of these cells in the healing (scar tissue formation) following isoproterenol-induced myocardial injury in rats. Alphasmooth muscle actin (α-SMA) expressing myofibroblasts were seen at the border of the affected area and appeared in the greatest numbers on days 3–7 post-injection, followed by a gradual decrease by day 35. The peak on day 3 was consistent with the timing of the highest proliferative activity of myofibroblasts. The number of ED1-positive macrophages began to increase as early as day 1, reaching a peak on day 3 within the injured myocardium. The expansion of EDI-positive macrophages preceded an increased number of α-SMA-positive myofibroblasts suggesting that myofibroblast proliferation and activation may be mediated by factors released by ED1-positive mcrophages in response to myocardial injury. The number of ED2-positive tissue-fixed, resident macrophages gradually increased from day 3 post-injection, and peaked on day 14, but the number of ED2-positive macrophages was consistently fewer than that of ED1-positive macrophages during the 35 day-observation period after the injection. The labelling index of the ED2-positive cells was maximal on day 14, indicative of local proliferation of resident macrophages. In the healing process after myocardial injury, EDI-positive macrophages increase markedly in the early stages; ED2-positive macrophages appear later.
Similar content being viewed by others
References
Adamson IYR, Letourneau HL, Bowden DH (1989) Enhanced macrophage-fibroblast interactions in the pulmonary interstitium increases fibrosis after silica injection to monocyte-depleted mice. Am J Pathol 134:411–418
Adler KB, Low RB, Leslie KO, Mitchell J, Evans JN (1989) Contractile cells in normal and fibrotic lung. Lab Invest 60:473–485
Amberger A, Bauer H, Tontsch U, Gabbiani G, Kocher O, Bauer HC (1991) Reversible expression of sm a-actin protein and sm α-actin mRNA in cloned cerebral endothelial cells. FEBS Lett 287:223–225
Bachem MG, Sell KM, Melchior R, Kropf J, Eller T, Gressner AM (1993) Tumor necrosis factor alpha (TNF α) and transforming growth factor β1 (TGF β1) stimulate fibronectin synthesis and the transdifferentiation of fat-storing cells in the rat liver into myofibroblasts. Virchows Arch [B] 63:123–130
Beelen RHJ, Eestermans IL, Döpp EA, Dijkstra CD (1987) Monoclonal antibodies ED I, ED2, and ED3 against rat macrophages: expression of recognized antigens in different stages of differentiation. Transplant Proc 19:3166–3170
Darby I, Skalli O, Gabbiani G (1990) α-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63:21–29
Desmoulière A, Rubbia-Brandt L, Grau G, Gabbiani G (1992) Heparin induces α-smooth muscle actin expression in cultured fibroblasts and in granulation tissue myofibroblasts. Lab Invest 67:716–726
Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-ß1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122: 103–111
Dijkstra CD, Döpp EA, Joling P, Kraal G (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54: 589–599
Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO (1987) Role of fibroblasts in accumulation of collagens in growing and adult rat hearts (abstract). J Mol Cell Cardiol 19 [Suppl IV]:S54
Eghbali M, Blumenfeld OO, Seifter S, Buttrick PM, Leinwand LA, Robinson TF, Zern MA, Giambrone MA (1989) Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol 21:103–113
Fishbein MC, Maclean D, Maroko PR (1978) The histopathologic evolution of myocardial infarction. Chest 73:843–849
Foo ITH, Naylor IL, Timmons MJ, Trejdosiewicz LK (1992) Intracellular actin as a marker for myofibroblasts in vitro. Lab Invest 67:727–733
Furth R van (1989) Origin and turnover a monocytes and macrophage. Curr Top Pathol 78:125–150
Goto M, Matsuno K, Yamaguchi Y, Ezaki T, Ogawa M (1993) Proliferation kinetics of macrophage subpopulations in a rat experimental pancreatitis model. Arch Histol Cytol 56:75–82
Hart DNJ, Fabre JW (1981) Demonstration and characterization of la-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med 153:347–361
Heuff G, Ende MB van der, Boutkan H, Prevoo W, Bayon LG, Fleuren GJ, Beelen RHJ, Meijer S, Dijkstra CD (1993) Macrophage populations in different stages of induced hepatic metastases in rats: an immunohistochemical analysis. Scand J Immunol 38:10–16
Hines JE, Johnson SJ, Burt AD (1993) In vivo responses of macrophages and perisinusoidal cells to cholestatic liver injury. Am J Pathol 142:511–518
Hinglais N, Heudes D, Nicoletti A, Mandet C, Laurent M, Bariéty J, Michel JB (1994) Colocalization of myocardial fibrosis and inflammatory cells in rats. Lab Invest 70:286–294
Hunt TK, Knighton DR, Thakral KK, Goodson WH III, Andrews WS (1984) Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery 96:48–54
Johnson SJ, Hines JE, Burt AD (1992) Macrophage and perisinusoidal cell kinetics in acute liver injury. J Pathol 166:351–358
Johnson SJ, Hines JE, Burt AD (1992) Immunolocalization of proliferating perisinusoidal cells in rat liver. Histochem J 24:67–72
Kovacs EJ (1991) Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today 12:17–23
Leibovich SJ, Ross R (1975) The role of the macrophage in wound repair: a study with hydrocortisone and antimacrophage serum. Am J Pathol 78:71–100
Leslie KO, Taatjes DJ, Schwarz J, Turkovich M von, Low RB (1991) Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol 139:207–216
Nagaoka I, Trapnell BC, Crystal RG (1990) Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest 85:2023–2027
Pierce GF, Vande Berg J, Rudolph R, Tarpley J, Mustoe TA (1991) Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol 138:629–646
Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS (1986) Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 83:4167–4171
Rona G, Chappel CI, Balazs T, Gaudry R (1959) An infarctlike myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol 67:99–111
Sappino AP, Schürch W, Gabbiani G (1990) Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 63:144–161
Schlingemann RO, Rietveld FIR, Kwaspen F, Kerkhof PCM van de, Waal RMW de, Ruiter DJ (1991) Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol 138:1335–1347
Scotti TM (1977) Rheumatic fever and rheumatic heart disease. In: Anderson WAD, Kissane JM (eds) Pathology. Mosby, Saint Louis, pp 779–793
Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G (1986) A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103:2787–2796
Skalli O, Schürch W, Seemayer T, Lagacé R, Montandon D, Pittet B, Gabbiani G (1989) Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest 60:275–285
Spencer SC, Fabre JW (1990) Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J Exp Med 171:1841–1851
Thompson NL, Bazoberry F, Speir EH, Casscells W, Ferrans VJ, Flanders KC, Kondaiah P, Geiser AG, Sporn MB (1988) Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors 1:91–99
Vracko R, Thorning D (1991) Contractile cells in rat myocardial scar tissue. Lab Invest 65:214–227
Vracko R, Thorning D, Frederickson RG (1989) Connective tissue cells in healing rat myocardium: a study of cell reactions in rhythmically contracting environment. Am J Pathol 134:993–1006
Willems IEMG, Havenith MG, De Mey JGR, Daemen MJAP (1994) The α-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol 145:868–875
Zhang J, Herman EH, Ferrans VJ (1993) Dendritic cells in the hearts of spontaneously hypertensive rats treated with doxorubicin with or without ICRF-187. Am J Pathol 142:1916–1926
Zhang J, Yu ZX, Fujita S, Yamaguchi ML, Ferrans VJ (1993) Interstitial dendritic cells of the rat heart: quantitative and ultrastructural changes in experimental myocardial infarction. Circulation 87:909–920
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakatsuji, S., Yamate, J., Kuwamura, M. et al. In vivo responses of macrophages and myofibroblasts in the healing following isoproterenol-induced myocardial injury in rats. Virchows Archiv 430, 63–69 (1997). https://doi.org/10.1007/BF01008018
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01008018