Abstract
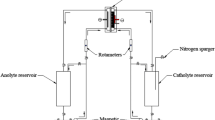
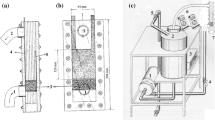
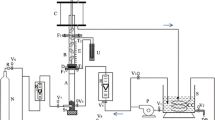
Unsteady state changes in ion concentration, liquid conductivity and electric current via different pathways in a bipolar packed-bed electrode cell were investigated by using the electrochemical reduction of Cu(II) ions. Ferrite pellets were used as particle electrodes and were packed in layers separated by plastic spacers. The electrode reaction rate was controlled by the diffusion of Cu(II) ions.
The faradaic current passing through the pellets decreased with time, while the bypass current through the liquid bulk phase increased with time because of the production of hydrogen ions. The faradaic and bypass currents and the overall current efficiency were well simulated by a proposed reactor model.
The overall current efficiency unavoidably decreased with time when the electrolyte was recirculated. However, the employment of trickle flow reduced the liquid hold-up in the bed and consequently reduced the bypass current. The overall current efficiency in the trickle-bed electrode cell was 20–30% higher than that in the flooded packed-bed electrode cell.
Similar content being viewed by others
Abbreviations
- A b :
-
resistance coefficient for bypass current (m−1)
- A s :
-
resistance coefficient for total current flowing through spacer region (m−1)
- C C :
-
concentration of Na2SO4 (mol m−3)
- C e :
-
overall current efficiency
- C i :
-
concentration of Cu2+ inith cell, function of time (mol m−3)
- C 0 :
-
concentration of Cu2+ in reservoir, function of time (mol m−3)
- C 0(0):
-
initial value ofC 0 (mol m−3)
- d :
-
diameter of pellet (m)
- D :
-
diffusion coefficient (m2 s−1)
- e i :
-
potential difference between upper and lower ends of each pellet (V)
- E :
-
cell voltage (V)
- F :
-
Faraday constant (C mol−1)
- I b :
-
mean value ofI bi (A)
- I bi :
-
bypass current inith cell, function of time (A)
- I p :
-
mean value ofI p (A)
- I pi :
-
faradaic current inith cell, function of time (A)
- I t :
-
total current (A)
- k :
-
mass transfer coefficient (m s−1)
- L T :
-
distance between two feeder electrodes (m)
- n :
-
number of pellet layers
- Q :
-
volumetric flow rate of electrolyte (m3 s−1)
- R bi :
-
equivalent resistance for bypass current inith cell, function of time (Ω)
- R pi :
-
equivalent resistance for faradaic current inith cell, function of time (Ω)
- R si :
-
equivalent resistance for total current inith cell, function of time (Ω)
- Re d :
-
Reynolds number,du 1/v 1
- Sc :
-
Schmidt number,v 1/D
- Sh d :
-
Sherwood number,kd/D
- S p :
-
total surface area of cathodic part of bipolarized pellets in each layer, defined by Equation 9 (m2)
- S T :
-
cross-sectional area of column (m2)
- t :
-
time (s)
- u 1 :
-
superficial liquid velocity (m s−1)
- V r :
-
volume of reservoir (m3)
- z :
-
number of electrons transferred in electrochemical reaction
- ɛ1 :
-
liquid holdup
- κ:
-
mean value of κi (S m−1)
- κi :
-
electrical conductivity of electrolyte inith cell, function of time (S m−1)
- κ(0):
-
initial value of κ (S m−1)
- λ:
-
molar conductivity (S mol−1 m−2)
- v 1 :
-
kinematic viscosity (m2 s−1)
- A:
-
CuSO4
- B:
-
H2SO4
- C:
-
Na2SO4
References
A. B. Smith and M. J. Hayes, Ger. Offen. 1949129, 9 April (1970).
M. Fleischmann, J. W. Oldfield and C. L. K. Tennakoon, Symposium on Electrochemical Engineering, University of Newcastle-upon-Tyne, Vol. 1 (1971) p. 53.
F. Goodridge, C. J. H. King and A. R. Wright,Electrochim. Acta 22 (1977) 347.
K. Kusakabe, S. Morooka and Y. Kato,J. Chem. Eng. Japan 15 (1982) 45.
K. Kusakabe, T. Kimura, S. Morooka and Y. Kato,17 (1984) 293.
K. Kusakabe, S. Morooka and Y. Kato,19 (1986) 43.
A. V. Boussoulengas, S. Ehdaie and R. E. W. Jansson,Chem. and Ind. Oct. (1979) 670.
M. Fleischmann and Z. Ibrisagic,J. Appl. Electrochem. 10 (1980) 151.
K. G. Ellis and R. E. W. Jansson,11 (1981) 531.
S. Ehadaie, M. Fleischmann and R. E. W. Jansson,12 (1982) 59.
E. A. El-Ghaoui, R. E. W. Jansson and A. R. Wright,12 (1982) 69.
E. A. El-Ghaoui and R. E. W. Jansson,12 (1982) 75.
F. Goodridge, C. J. H. King and A. R. Wright,Electrochim. Acta 22 (1977) 1087.
C. J. H. King, K. Lister and R. E. Plimley,Trans. Instn Chem. Engrs 53 (1977) 20.
E. J. Wilson and C. J. Geankoplis,Ind. Eng. Chem. Fundam. 5 (1966) 9.
D. A. G. Bruggeman,Ann. Phisik. 24 (1935) 636.
J. Newman, ‘Electrochemical Systems’, Prentice Hall (1973) p. 364.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kusakabe, K., Kimura, T., Morooka, S. et al. Recirculating feed operation of bipolar packed-bed and trickle-bed electrode cells equipped with mesh spacers. J Appl Electrochem 17, 724–730 (1987). https://doi.org/10.1007/BF01007807
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01007807