Abstract
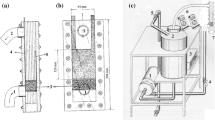
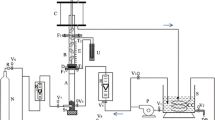
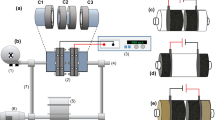
Mass transport properties of a flow-by fixed bed electrochemical reactor composed of a vertical stack of stainless steel nets operated at a batch-recycle mode were characterized using cathodic deposition of copper as a test reaction. The electrochemical reactor was operated at constant potential in which reduction of copper happened under mass transport control. This potential was selected from the application of hydrodynamic voltammetry using a borate/chloride solution as supporting electrolyte on stainless steel rotating disc electrode. A linear relationship was observed between the flow rate and the mass transfer coefficient. The electrochemical reactor was efficient in removing copper and able to reduce the levels of this metal to lower than 0.4 ppm starting from an initial concentration of 49.7 ppm at 80 min using a ratio of cathode volume/catholyte volume equal to 0.0075. A mathematical correlation between the Sherwood number and Reynolds number were obtained which characterized the mass transport properties of the reactor as follows: Sh = 0.2254Re0.4228Sc1/3.






Similar content being viewed by others
Abbreviations
- a :
-
Specific surface area cm2 cm−3
- A :
-
Cross sectional area of electrode cm2
- b :
-
Power of Reynolds Number in Eq. 10 –
- B :
-
Opening size of screen cm
- C(0) :
-
Initial Concentration at time = 0 mol.cm−3 or ppm
- C(t) :
-
Concentration at time = t mol.cm−3 or ppm
- Cb :
-
Bulk concentration mol.cm−3 or ppm
- d :
-
Diameter of wire screen cm
- D :
-
Diffusivity cm2 s−1
- E :
-
Cell potential mV
- Emax :
-
Maximum electrode potential mV
- Emin :
-
Minimum electrode potential mV
- F :
-
Faraday number(96500) C mo1−1
- i :
-
Current density mA cm−2
- ilm :
-
Limiting current density mA cm−2
- IL :
-
Limiting current mA
- jD :
-
Chilton-Colburn j-factor –
- km :
-
Mass transfer coefficient cm s−1
- L :
-
Length of electrode cm
- Li :
-
Length of wire segment in Eq. (3-c) cm
- m :
- N :
-
Mesh size number Wire/in.
- n :
-
Power of Reynolds Number in Eq. 11 –
- Q :
-
Volumetric Flow rate cm3 s−1 or dm3 h−1
- t :
-
Time s
- u :
-
Flow velocity of solution m s−1
- Vr :
-
Volume of reservoir cm3
- w :
-
Thickness of electrode cm
- z :
-
Number of electrons –
- Re :
-
Reynolds number (Re = ud/ ε ν) –
- Sh :
-
Sherwood number dimensionless(Sh = km d/D) –
- Sc :
-
Schmidt number dimensionless(Sc = μ/ρD) –
- X :
-
Geometric dimensionless parameter –
- ρ :
-
Fluid density g cm−3
- μ :
-
Viscosity of fluid g m−1 s−1
- ν :
-
Kinematic viscosity of fluid cm2 s−1
- ε :
-
Void fraction or porosity –
- ω :
-
Rotation velocity rad s−1
References
Pletcher D, Walsh FC (1990) Industrial electrochemistry, 2nd edn. Chapman and Hall, London
Bourgeois W, Burgess JE, Stuets RM (2001) On-line monitoring of wastewater quality: a review. J Chem Technol Biotechnol 76:337–348
Chen JP, Wang X (2000) Removing copper, zinc, and lead ion by granular activated carbon in pretreated fixed-bed columns. Sep Purif Technol 19:157–167
Ismail IM, El-Sourougy MR, Abdel Moneim N, Aly HF (1999) Equilibrium and kinetic studies of the sorption of cesium by potassium nickel hexacyanoferrate complex. J Radioanal Nucl Chem 240:59–67
Kazemipour M, Ansari M, Tajrobehkar SH (2008) Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J Hazard Mater 150:322–327
Tenorio JAS, Espinosa DCR (2001) Treatment of chromium plating process effluents with ion exchange resins. Waste Manag 21:637–642
Mishraa PC, Patel RK (2009) Removal of lead and zinc ions from water by low cost adsorbents. J Hazard Mater 168:319–325
Alizadeh T, Amjadi S (2011) Preparation of nano-sized Pb2+ imprinted polymer and its application as the chemical interface of an electrochemical sensor for toxic lead determination in different real samples. J Hazard Mater 190:451–459
Khajeha M, Heidarib ZS, Sanchoolia E (2011) Synthesis, characterization and removal of lead from water samples using lead-ion imprinted polymer. Chem Eng J 166:1158–1163
Mohsen-Nia M, Montazeri P, Modarress H (2007) Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 217:276–281
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41
Juttner K, Galla U, Schmieder H (2000) Electrochemical approaches to environmental problems in the process industry. Electrochim Acta 45:2575–2594
Ismail IM, Abdel-Salam OE, Ahmed TS (2013) Investigation of the anodic dissolution of zinc in sodium chloride electrolyte – a green process. Port Electrochim Acta 31:207–219
Fedkiw PS (1981) Ohmic potential drop in flow-through and flow-by porous electrodes. J Electrochem Soc 128:831–838
Walsh FC (1993) A first course in electrochemical engineering. The Electrochemical Consultancy, Hampshire
Storck A, Robertson PM, Ibl N (1979) Mass transfer study of three-dimensional electrodes composed of stacks of nets. Electrochim Acta 24:373–380
Leroux F, Coeuret F (1985) Flow-by electrodes of ordered sheets of expanded metal I. Current distribution for diffusional regime. Electrochim Acta 30:159–166
Leroux F, Coeuret F (1985) Flow-by electrodes of ordered sheets of expanded metal II. Potential distribution for diffusional regime. Electrochim Acta 30:167–172
Simonsson D (1984) A flow-by packed-bed electrode for removal of metal ions from waste water. J Appl Electrochem 14:595–604
Montillet A, Comiti J, Legrand J (1993) Application of metallic foams in electrochemical reactors of filter-press type part I: flow characterization. J Appl Electrochem 23:1045–1052
Wang J (1981) Reticulated vitreous carbon—a new versatile electrode material. Electrochim Acta 26:1721–1726
Shah AA, Al-Fetlawi H, Walsh FC (2010) Dynamic modelling of hydrogen evolution effects in the all-vanadium redox flow battery. Electrochim Acta 55:1125–1139
Ferreira BK (2008) Three-dimensional electrodes for the removal of metals from dilute solutions: a review. Min Proc Ext Met Rev 29(4):330–371
Abdel Aziz MH, Nirodosh I, Sedahmed GH (2012) Mass transfer at vertical oscillating screen stack in relation to catalytic and electrochemical reactor design. Ind Eng Chem Res 51:11636–11642
Zaki MM, Nirodosh I, Sedahmed GH (2007) Mass transfer characteristics of reciprocating screen stack electrochemical reactor in relation to heavy metal removal from dilute solutions. Chem Eng J 126:67–77
Sioda RE (1974) Application of flow electrolysis on porous electrodes for electropreparations. J Electroanal Chem Interfacial Electrochem 56:149–154
Abbar AH, Salman RH, Abbas AS (2018) Studies of mass transfer at a spiral-wound woven wire mesh rotating cylinder electrode. Chem Eng Process Process Intensif 127:10–16
Goodridge F, Scott K (1995) Electrochemical process engineering, a guide to the design of electrolysis plant. Plenum Press, New York
Sioda RE (1978) Criterion of completeness of electrolysis at flow porous electrodes. J Appl Electrochem 8:297–304
Nava JL, Sosa E, Carreno G, Ponce-de-leon C, Oropeza MT (2006) Modelling of the concentration–time relationship based on global diffusion-charge transfer parameters in a flow-by reactor with a 3D electrode. Electrochim Acta 51:4210–4217
Walker ATS, Wragg AA (1977) The modelling of concentration–time relationships in recirculating electrochemical reactor systems. Electrochim Acta 22:1129–1134
Atlas H, Resnick W (1976) Analysis of batch-recycle reactor systems. Can J Chem Eng 54:639–641
Mobarak AA, Abdo MSE, Hassan MSM, Sedahmed GH (2000) Mass transfer behavior of a flow-by fixed bed electrochemical reactor composed of a vertical stack of screens under single and upward two phase flow. J Appl Electrochem 30:1269–1276
Cano J, Bohm U (1977) Mass transfer in packed beds of screens. Chem Eng Sci 32:213–219
Sioda RE (1977) Axial dispersion in flow porous electrodes. J Appl Electrochem 7:135–137
Sioda RE (1977) Flow-through electrodes composed of parallel screens. Electrochim Acta 22:439–443
Sioda RE (1976) Mass transfer problems in electrolysis with flowing solution on single and stacked screens. J Electroanal Chem 70:49–54
Vogtlander PH, Bakker CAP (1963) An experimental study of mass transfer from a liquid flow to wires and gauzes. Chem Eng Sci 18:583–589
Paidar M, Bouzek K, Laurich M, Thonstad J (2000) Application of a three-dimensional electrode to the electrochemical removal of copper and zinc ions from diluted solutions. Water Environ Res 72:618–625
Findlay A, Kiitchener JK (1965) Practical physical chemistry. Longmans, London
Risch T, Newman J (1984) A theoretical comparison of flow-through and flow-by porous electrodes at the limiting current. J Electrochem Soc 131:2551–2565
Armour JC, Cannon JN (1968) Fluid flow through woven screens. AICHE J 14:415–420
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications. Wiley, New York
Quickenden TI, Xu Q (1996) Toward a reliable value for the diffusion coefficient of cupric ion in aqueous solution. J Electrochem Soc 143:1248–1253
Ponce de-Leon C, Low CTJ, Kear G, Walsh FC (2007) Strategies for the determination of the convective-diffusion limiting current from steady state linear sweep voltammetry. J Appl Electrochem 37:1261–1270
Pletcher D (1991) A first course in electrode processes. the Electrochemical Consultancy, Romsey
Pletcher D, Whyte I, Walsh FC, Millington JP (1991) Reticulated vitreous carbon cathodes for metal ion removal from process streams part I: mass transport studies. J Appl Electrochem 21:659–666
Ford WPJ, Walsh F, Whyte I (1992) Simplified batch reactor models for the removal of metal ions from solution. I Chem E Symp Ser 127:111–126
Stankovic VD, Wragg AA (1995) Modelling of time-dependent performance criteria in a three-dimensional cell system during batch recirculation copper recovery. J Appl Electrochem 25:565–573
Rajesshwar K, Ibanez JG (1997) Environmental electrochemistry: fundamentals and applications in pollution sensors and pollutant treatment. Academic, San Diego
Shah MA, Roberts D (1974) Mass transfer characteristics of stacked metal screens. Advances in Chemistry, vol 133, Chemical Reaction Engineering-II, chapter 20:259–270
Hor YP, Mohamed N (2003) Removal and recovery of copper via a galvanic cementation system part I: single-pass reactor. J Appl Electrochem 33:279–285
Hor YP, Mohamed N (2005) Removal and recovery of copper via a galvanic cementation system part II: batch recycle reactor. J Appl Electrochem 35:609–613
Pletcher D, Whyte I, Walsh FC, Millington JP (1993) Reticulated vitreous carbon cathodes for metal ion removal from process streams part II. Studies of a single pass reactor. J Appl Electrochem 23:82–85
Acknowledgments
The authors express their gratitude to Engineering Consulting Bureau/ Al-Qadissya University for financial support of this work under the contract no.28-2. Special thanks are also due to the technical staff of Chemical Engineering Department, University of Baghdad for their support and assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbar, A.H., Sulaymon, A.H. & Mohammed, S.A.M. Mass transfer characteristics of a flow-by fixed bed electrochemical reactor composed of vertical stack stainless steel screens cathode. Heat Mass Transfer 55, 2419–2428 (2019). https://doi.org/10.1007/s00231-019-02591-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-019-02591-4