Abstract
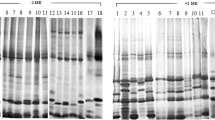
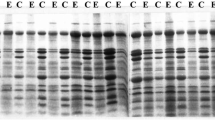
Seed proteins fromCicer arietinum L.,C. reticulatum Ladiz. andC. echinospermum Davis were extracted and separated into water soluble (albumin) and water insoluble (globulin) fractions. These were analysed using three polyacrylamide gel systems: uniform pore slab gels, gradient gels and SDS disc gels. For all three species, albumins constitute just over one-third of total protein. Minor differences in the composition of this fraction were observed. Within the globulin fraction, seven disulphide-linked polypeptides were found. Four of these resemble the major polypeptide of legumin, consisting of constant small subunit (21,000 daltons) linked to variable large subunit (46,000, 41,000, 39,000 or 36,000 daltons), forming polypeptides of 67,000 (I), 62,000 (II), 60,000 (III) and 57,000 (IV) daltons respectively. Polypeptide I was prominent in both wild species, but absent fromC. arietinum. Polypeptides II and III were equally prominent inC. arietinum andC. reticulatum. Polypeptide IV was more prominent inC. echinospermum, which was deficient in polypeptide III. Polypeptides V (45,000 daltons) and VI (43,000 daltons), apparently composed of two equal subunits, were present in trace amounts in both wild species, but well represented inC. arietinum Polypeptide VII of 45,000 daltons (31,000 + 14,000) was present in all three species.
Similar content being viewed by others
References
Clarke, G. C. S., Kupicha, F. K., 1976: The relationships of the genusCicer L. (Leguminosae): the evidence from pollen morphology. — Bot. J. Linn. Soc.72, 35–44.
Davis, P. H., 1970:Cicer L. — In:Davis, P. H., (Ed.): Flora of Turkey3, pp. 267–274. — Edinburgh: University Press.
DeJong, W. W., Zweers, A., Cohen, L. H., 1978: Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes.—Biochem. Biophys. Res. Commun.82, 532–539.
Dudman, W. F., Millerd, A., 1975: Immunochemical behaviour of legumin and vicilin fromVicia faba: a survey of related proteins in the subfamilyFaboideae. — Biochem. System. Ecol.3, 25–33.
Ganesh Kumar, K., Venkataraman, L. V., 1980: Chickpea seed proteins: isolation of and characterization of 10.3 S protein. — J. Agr. Food Chem.28, 524–529.
Hedrick, J. L., Smith, A. J., 1968: Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. — Arch. Biochem. Biophys.126, 155–164.
Jackson, P., Boulter, D., Thurman, D. A., 1969: A comparison of some properties of vicilin and legumin isolated from seeds ofPisum sativum, Vicia faba, andCicer arietinum — New Phytol.68, 25–33.
Kupicha, F. K., 1977: The delimitation of the tribeVicieae (Leguminosae) and the relationships ofCicer L. — Bot. J. Linn. Soc.74, 131–162.
, 1981:Cicereae. In:Polhill, R. M., Raven, P. H., (Eds.): Advances in Legume Systematics, Part 1, p. 377. Royal Botanic Gardens, Kew, U.K.
Ladizinsky, G., 1975: A new wildCicer from Turkey. — Notes Royal Bot. Gard. Edinburgh31, 201–202.
, 1975: The origin of chick pea as indicated by seed protein electrophoresis. — Israel J. Bot.24, 183–189.
, 1976: The origin of chickpeaCicer arietinum L. — Euphytica25, 211–217.
, 1976: Genetic relationships among the annual species ofCicer L. — Theor. Appl. Genet.48, 197–203.
Lersten, N. R., Gunn, C. R., 1981: Seed morphology and testa topography inCicer (Fabaceae: Faboideae).—System. Bot.6, 223–230.
Murray, D. R., 1979a: A storage role for albumins in pea cotyledons. — Plant Cell Environ.2, 221–226.
, 1979b: The seed proteins of Kowhai,Sophora microphylla Ait. — Z. Pflanzenphysiol.93, 423–428.
, 1979c: Proteolysis in the axis of the germinating pea seed. II. Changes in polypeptide composition. — Planta147, 117–121.
, 1980: A comparative electrophoretic study of the seed albumins fromSophora microphylla andPisum sativum (Leguminosae). — Pl. System. Evol.134, 207–214.
, 1982a: Albumins from pea seeds are distinct from vicilin. — Z. Pflanzenphysiol.106, 465–468.
, 1982b: The occurrence of disulphide-linked polypeptides in helianthin, the major reserve globulin of sunflower seed. — Z. Pflanzenphysiol.108, 181–185.
, 1978: Comparative biochemical and morphological studies ofAcacia sophorae (Labill.)R. Br. andA. longifolia (Andrews)Willd. — Aust. J. Bot.26, 755–771.
Spencer, D., Higgins, T. J. V., Button, S. C., Davey, R. A., 1980: Pulselabelling studies on protein synthesis in developing pea seeds and evidence of a precursor form or legumin small subunit. — Pl. Physiol.66, 510–515.
Thomson, J. A., Schroeder, H. E., Dudman, W. F., 1978: Cotyledonary storage proteins inPisum sativum I. Molecular heterogeneity. — Aust. J. Pl. Physiol.5, 263–279.
Vairinhos, F., Murray, D. R., 1982a: Changes in polypeptide composition during seed development in chickpea,Cicer arietinum L. — Z. Pflanzenphysiol.106, 447–452.
, 1982b: Variation in the sizes of both large and small disulphide-linked subunits of legumin in representatives ofVicieae andCicer. — Z. Pflanzenphysiol.107, 25–32.
Weber, K., Osborn, M., 1969: The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. — J. Biol. Chem.244, 4406–4412.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vairinhos, F., Murray, D.R. The seed proteins of chick pea: Comparative studies ofCicer arietinum, C. reticulatum andC. echinospermum (Leguminosae) . Pl Syst Evol 142, 11–22 (1983). https://doi.org/10.1007/BF00989600
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00989600