Abstract
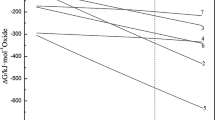
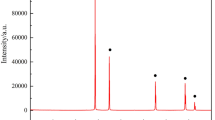
The mechanism of the reduction of carbon/alumina powder mixture in a flowing nitrogen stream was studied. Five steps were found to be involved in the overall reaction.
The consumption rates of Al2O3 and carbon, and the production rate of AIN, were determined to be
in the temperature range 1648–1825 K.
Similar content being viewed by others
References
N. Kuramoto andH. Taniguchi, US Pat. 4618 592 (1986).
H. L. Wang, MS thesis, Department of Mineral Metallurgy and Materials Science, National Cheng Kung University, Tainan, Taiwan (1988).
S. Hirai, T. Miwa, M. Ozawa andH. G. Katayama,J. Jpn Inst. Metals 53 (1989) 1035.
H. Inoue, A. Tsung andM. Kasori,J. Mater. Sci. 25 (1990) 2359.
C. I. Lin andC. Lee, Technical Report to National Science Council of Taiwan, NSC81-0402-E011-02, Taipei, Taiwan (1992).
A. G. Vodopsanov, A. V. Serebryakova andG. N. Kozhevnikov,Izv. Akad. Nauk SSSR Met. 1 (January–February) (1982) 43.
T. Sakai andM. Iwata,J. Ceram. Soc. Jpn 82 (3) (1974) 41.
Y. W. Cho andT. A. Charles,Mater. Sci. Technol. 7 (1991) 495.
L. Brewer,J. Am. Chem. Soc. 73 (1951) 5308.
Y. K. Rao andB. P. Jalan,Metall. Trans. 3 (1972) 2465.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, H.K., Lin, C.I. Mechanism of the reduction of carbon/alumina powder mixture in a flowing nitrogen stream. J Mater Sci 29, 1352–1357 (1994). https://doi.org/10.1007/BF00975088
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00975088