Abstract
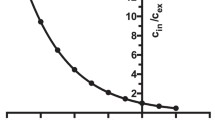
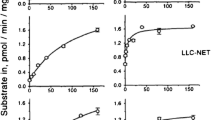
The association of [3H]-Met-enkephalin with synaptosomes isolated from rat brain cortex, when incubated for 30 min at 25°C follows a sigmoid path with a Hill coefficient h=1.25±0.04. Binding of Met-enkephalin into synaptosomes was saturable, with an apparent binding constant of 8.33±0.48 nM. At saturation, Met-enkephalin specific receptors corresponded to 65.5±7.2 nmol/mg synaptosomal protein. The Hill plot in combination with the biphasic nature of the curve to obtain the equilibrium constant, showed a moderate degree of positive cooperativity in the binding of Met-enkephalin into synaptosomes of at least one class of high affinity specific receptors. Met-enkephalin increased the lipid fluidity of synaptosomal membranes labelled with 1,6-diphenyl-1,3,5-hexatriene (DPH), as indicated by the steady-state fluorescence anisotropy [(ro/r)−1]−1. Arthenius-type plots of [(ro/r)−1]−1 indicated that the lipid separation of the synaptosomal membranes at 23.4±1.2°C was perturbed by Met-enkephalin such that the temperature was reduced to 15.8±0.8°C. Naloxone reversed the fluidizing effect of Met-enkephalin, consistent with the receptor-mediated modulation of membrane fluidity. Naloxone alone had no effect on membrane fluidity. NO release and cGMP production by NO-synthase (NOS) and soluble guanylate cyclase (sGC), both located in the soluble fraction of synaptosomes (synaptosol) were decreased by 82% and 80% respectively, after treatment of synaptosomes with Met-enkephalin (10−10–10−4 M). These effects were reversed by naloxone (10−4 M) which alone was ineffective in changing NO and cGMP production. We propose that Met-enkephalin achieved these effects through receptor mediated perturbations of membrane lipid structure and that inhibition of the L-Arg/NO/cGMP pathway in the brain may result in the antinociceptive effects of Met-enkephalin.
Similar content being viewed by others
References
Southan, E., and Garthwaite, J., 1993. The nitric oxide-cGMP signaling pathway in rat brain. Neuropharmacology 32:1267–1277.
Bruhwyler, J., Chleide, E., Liegeois, J. F., and Career, F., 1993. Nitric oxide: a new messenger in the brain. Neurosci. Biobehav. Rev. 17:373–384.
Deliconstantinos, G., Villiotou, V., and Stavrides, J. C., 1994. Pathophysiology of nitric oxide (NO) in cancer. Cancer Mol. Biol. 1:77–86.
Klatt, P., Schmidt, K., Brunner, F., and Mayer, B., 1994. Inhibitors of brain nitric oxide synthase. Binding kinetics, metabolism and enzyme inactivation. J. Biol. Chem. 269:1674–1680.
Bansinath, M., Arbabha, B., Turndorf, H., and Garg, V. C., 1993. Chronic administration of a nitric oxide synthase inhibitor, N-nitro-L-arginine, and drug-induced increase in cerebella cGMP in vivo. Neurochem. Res. 18:1063–1066.
Buras, B., Izenwasser, S., Portoghese, P. S., and Cox, B. M., 1994. Evidence for delta opioid receptor subtypes regulating adenylyl cyclase activity in rat brain. Life Sci. 54:PL 101–106.
Selley, D. E., Breivogel, C. S., and Childers, S. R., 1993. Modification of G protein-coupled functions by low-pH pretreatment of membranes from NG 108-15 cells: increase in opioid agonist efficacy by decreased inactivation of G proteins. Mol. Pharmacol. 44:731–741.
Papaphilis, A., and Deliconstantinos, G., 1980. Modulation of serotonergic receptors by exogenous cholesterol in the dog brain synaptosomal plasma membranes. Biochem. Pharmacol. 29:3325–3327.
Deliconstantinos, G., Kopeikina-Tsiboukidou, L., and Villiotou, V., 1987. Opiate receptors mediated increase of membrane fluidity modulates the major enzymes of the second messenger pathways in rat brain synaptosomal plasma membranes. Anticancer Res. 7: 915–916.
Kopeikina-Tsiboukidou, L., and Deliconstantinos, G., 1989. Calmodulin selectively modulates the guanylate cyclase activity by repressing the lipid phase separation temperature in the inner half of the bilayer of rat brain synaptosomal plasma membranes. Neurochem. Res. 14:119–127.
Slater, S. J., Kelly, M. B., Taddeo, F. J., Ho, G., Rubin, E., and Stubbs, C. D. 1994. The mo lulation of protein kinase C activity by membrane lipid bilayer structure. J. Biol. Chem. 269:4866–4871.
Deliconstantinos, G., and Villiotou, V., 1992. Modulation of nitric oxide (NO) production by active oxygen species in keratinocytes, Pages 182–185,in: S. Moncada, M. A. Marletta, J. B. Hibbs Jr and E. A. Higgs (eds) Biology of nitric oxide” Portland Press.
Van der Vliet, A., and Bast, A., 1992. Effect of oxidative stress on receptors and signal transmission. Chem. Biol. Interact. 85:95–116.
Miller, G. L., 1959. Protein determination for large number of samples. Analyt. Chem. 31:964–968.
Deliconstantinos, G., Villiotou, V., and Fassitsas, Ch., 1992. Ultraviolet radiated human erdothelial cells elaborate nitric oxide that may evoke vasodilatory response. J. Cardiovasc. Pharmacol. 20:S63-S65.
Shinitzky, M., and Barenholz, Y., 1978. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta 515:367–394.
Deliconstantinos, G., 1986. Prostaglandin F2a binding on dog brain synaptosomal plasma membranes and its evoked effects on membrane fludity, (Na++K+)-stimulated ATPase and Ca2+-stimulated ATPase activities. Cell. Mol. Biol. 32:113–119.
Deliconstantinos, G., Kopeikina-Tsiboukidou, L., and Villiotou, V., 1989. Evoked effects of cholesterol binding on integral proteins and lipid fluidity of dog brain synaptosomal plasma membranes. Biochem. Cell. Biol. 67:16–24.
Scatchard, G., 1949. The attraction of protein for small molecules and ions. Ann. N.Y. Acad. Sci. 51:660–672.
Deliconstantinos, G., 1983. Phenobarbital modulates the (Na++K+)-stimulated ATPase and Ca2+-stimulated ATPase activities by increasing the bilayer fluidity of dog brain synaptosomal plasma membranes. Neurochem. Res. 8:1143–1152.
Akera, T., and Cheng, V. K., 1977. A simple method for the determination of affinity and binding site concentration in receptor binding studies. Biochim. Biophys. Acta 470:412–423.
Heron, D., Israeli, M., Hershkowitz, M., Samuel, D., and Shinitzky, M., 1981. Lipid-induced modulation of opiate receptors in mouse brain membranes. Eur. J. Pharmacol. 72:361–364.
Deliconstantinos, G., 1990. Effects of prostaglandin E2 and progesterone on rat brain synaptosomal plasma membrane. Ciba Foundation Symposium 153, Steroids and Neuronal Activity, pp 190–205, John Wiley and Sons, New York.
Deliconstantinos, G., 1991. Effect of anionic and cationic drugs on synaptosomal membrane fluidity, enzyme activity and transport, In “Advances in Membrane Fluidity” Drug and Anesthetic Effects on Membrane Structure and Function (R. C. Aloia, C. C. Curtain, L. M. Gordon) Vol. 5, pp. 131–149, Wiley-Liss.
Choi, D. W., 1993. Nitric oxide: Foe or friend to the injured brain. Proc. Natl. Acad. Sci. USA 90:9741–9743.
Kow, L. M., and Pfaff, D. W., 1988. Neuromodulatory actions of peptides. Ann. Rev. Pharmacol. Toxicol. 28:163–175.
Moncada, S., Palmer, R. M. J., and Higgs, E. A., 1991. Nitric Oxide: Physiology-pathophysiology and pharmacology. Pharmacological Rev. 43:109–142.
Myers, P. R., Minor, R. L. Jr, Guerra, R. Jr., Bates, J. N., and Harrison, D. G., 1990. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature (Lond.) 345:161–163.
Meller, S. T., and Gebhart, G. F., 1993. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain 52:127–136.
Kawabata, A., Nashimura, Y., and Takagi, H. 1992. L-leucyl-L-arginine, naltrindole and D-arginine block antinociception elicited by L-arginine in mice with carrageenin-induced hyperalgesia. Br. J. Pharmacol. 107:1096–1101.
Moore, P. K., Wallace, P., Gaffen, Z., Hart, S. L., and Babbedge, R. C., 1993. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles; antinociceptive and cardiovascular effects. Br. J. Pharmacol. 110:219–224.
Kawabata, A., Umeda, N., and Takagi, H., 1993. L-arginine exerts a dual role in nociceptive processing in the brain: involvement of the kyotorphin-Met-enkephalin pathway and NO-cGMP pathway. Br. J. Pharmacol. 109:73–79.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deliconstantinos, G., Villiotou, V. & Stavrides, J.C. Met-enkephalin receptor-mediated increase of membrane fluidity modulates nitric oxide (NO) and cGMP production in rat brain synaptosomes. Neurochem Res 20, 217–224 (1995). https://doi.org/10.1007/BF00970547
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00970547