Abstract
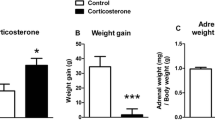
The aim of the present study was to investigate whether the hiopocampus exerts a modulatory effect on the activity of the hypothalamic-pituitary-adrenal (HPA) axis. Kainic acid was stereotaxically injected into the CA1 pyramidal cell layer of the dorsal hippocampus, causing histological and behavioural changes typical of kainic acid toxicity. The CA3 pyramidal cells of the dorsal hippocampus were selectively lesioned. Rats treated with kainic acid were hyperactive, executed clockwise rotatory movements and displayed epileptic seizures. The acute excitatory effect of kainic acid on glutamatergic receptors in the hippocampus resulted in an elevation in plasma corticosterone levels, suggesting a stimulation of HPA axis activity. Direct or indirect stimulation of the CA1 pyramidal cells of the dorsal hippocampus appeared to have caused the increase in corticosterone secretion.
Similar content being viewed by others
References
Lamberts, S. W. J., Bons, E., and Zuiderwijk, J. 1986. High concentrations of catecholamines selectively diminish the sensitivity of CRF-stimulated ACTH release by cultured rat pituitary cells to the suppressive effects of dexamethasone. Life Sci. 39:97–102.
Weizman, A., Gil-Ad, I., Grupper, D., Tyano, S., and Laron, Z. 1987. The effect of acute and repeated electroconvulsive treatment on plasma β-endorphin, growth hormone, prolactin and cortisol secretion in depressed patients. Psychopharmacol. 93:122–126.
De Villiers, A. S., Russell, V. A., Carstens, M. E. C., Aalbers, C., Gagiano, C. A., Chalton, D. O., and Taljaard, J. J. F. 1987. Noradrenergic function and hypothalamic-pituitary-adrenal axis activity in primary unipolar major depressive disorder. Psychiatry Res. 22:127–140.
Sachar, E. F. 1982. Endocrine abnormalities in depression. Pages 191–201,in Paykel, E. S. (Ed.), Handbook of Affective Disorders, Chuchill Livingstone, London.
Keller-Wood, M., Kimura, B., Shinsako, J., and Philips, M. I. 1986. Interaction between CRF and angiotensin-II in control of ACTH and adrenal steroids. Am. J. Physiol. 250:R396-R402.
Rivier, C. L., and Plotsky, P. M. 1986. Mediation by corticotropin-releasing factor of adenohypophysial hormone secretion. Ann. Rev. Physiol. 48:475–494.
Beaulieu, S., Di Paolo, T., Cote, J., and Barden, N. 1987. Participation of the central amygdaloid nucleus in the response of adrenocorticotropin secretion to immobilization stress: opposing roles of the noradrenergic and dopaminergic systems Neuroendocrinol. 45:37–46.
Makara, G. B. 1985. Mechanisms by which stressfull stimuli activate the pituitary-adrenal system. Fed. Proc. 44:149–153.
Gerlach, J. L., and McEwen, B. 1972. Rat brain binds adrenal steroid hormones: radioautography of hippocampus with corticosterone. Science 175:1133–1136.
Magarinos, A. M., Somoza, G., and de Nicola, A. F. 1987. Glucocorticoid negative feedback and glucocorticoid receptors after hippocampectomy in rats. Horm. Metab. Res. 19:105–109.
Sapolsky, R. M., Krey, L., and McEwen, B. 1984. Stress downregulates corticosterone receptors in a site specific manner in the brain. Endocrinology 114:287–292.
Agrawal, S. G., and Evans, R. H., 1986. The primary afferent depolarizing action of kainate in the rat. Br. J. Pharm. 87:345–355.
Harms, P. G., and Ojeda, S. R. 1974. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J. Appl. Physiol. 36:391–392.
Pellegrino, L. J., Pellegrino, S. A., and Cushman, A. J. 1979. A stereotaxic atlas of the rat brain. Plenum press, New York.
Al-Dujaili, E. A. S., Williams, B. C., and Edwards, C. R. W. 1981. The development and application of a direct radioimmunoassay for corticosterone. Steroids 37:157–176.
D'Agostino, J., Vaeth, G. F., and Henning, S. J. 1982. Diurnal rhythm of total and free concentrations of serum corticosterone in the rat. Acta Endocrinol. 100:85–90.
De Boer, S. F., and van der Gugten, J. 1987. Daily variations in plasma noradrenaline, adrenaline and corticosterone concentrations in the rat. Physiol. Behav. 40:323–328.
Munoz, C., and Grossman, S. P. 1980. Some behavioral effects of selective neuronal depletion by kainic acid in the dorsal hippocampus of rats. Physiol. Behav. 25:581–587.
Schwarcz, R., Zaczek, R., and Coyle, J. T. 1978. Microinjcction of kainic acid into the rat hippocampus. Eur. J. Pharmacol. 50:209–220.
Pohorecky, L. A., Cotler, S., Carbone, J. J. and Roberts, P. 1988. Factors modifying the effect of diazepam on plasma corticosterone levels in rats. Life Sci. 43:2159–67.
Meador-Woodruff, J. H., and Greden, J. F. 1988. Effect of psychotropic medications on hypothalamic-pituitary-adrenal regulation. Neurol. Clin. 6(1):225–34.
Manev, H., and Pericic, D. 1983. Hypothalamic GABA system and plasma corticosterone in ether stressed rats. Pharmacol. Biochem. Behav. 18(6):847–50.
Petraglia, F., Bakalakis, S., Facchinetti, F., Volpe, A., Muller, E. E., and Genazzani, A. R. 1986. Effects of sodium valproate and diazepam on beta-endorphin, beta-lipotropin and cortisol secretion induced by hypoglycemic stress in humans. Neuroendocrinol. 44(3):320–5.
Casady, R. L. and Taylor, A. N. 1976. Effect of electrical stimulation of the hippocampus upon corticosteroid levels in the freely-behaving, non-stressed rat. Neuroendocrinol. 20:68–78.
Feldman, S. 1985. Neural pathways mediating adrenocortical responses. Fed. Proc. 44:169–175.
Dunn, J. d., and Orr, S. E. 1984. Differential plasma corticosterone responses to hippocampal stimulation. Exp. Brain Res. 54:1–6.
Nadler, J. V., Perry, B. W., Gentry, C., and Cotman, C. W. 1980. Degeneration of hippocampal CA3 pyramidal cells induced by intraventricular kainic acid. J. Comp. Neurol. 192:333–359.
Berger, M. L., Charton, G., and Ben-Ari, Y. 1986. Effect of seizures induced by intra-amygdaloid kainic acid on kainic acid binding sites in rat hippocampus and amygdala. J. Neurochem. 47:720–727.
Tremblay, E., Repressa, A., and Ben-Ari, Y. 1985. Autoradiographic localization of kainic acid binding sites in the human hippocampus. Brain Res. 343:378–382.
Madison, D. V., and Nicoll, R. A. 1988. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 442:131–138.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Daniels, W.M.U., Jaffer, A., Engelbrecht, A.H. et al. The effect of intrahippocampal injection of kainic acid on corticosterone release in rats. Neurochem Res 15, 495–499 (1990). https://doi.org/10.1007/BF00966206
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00966206