Abstract
Metabolic characteristics of experimental hepatoma cells include elevated rates of glycolysis and lipid synthesis. However, pyruvate derived from glucose is not redily oxidized, and the source of acetly CoA for lipid synthesis in As-39D cells has not been characterized. In this study ketone bodies were examined as a possible source of acetyl CoA in AS-30D hepatoma cells.
The major findings were:
-
1.
Acetoacetate was utilized by AS-30D cells, with14C-lipid and14CO2 as major products of [3-14C] acetoacetate.
-
2.
Lipid synthesis from acetoacetate was dependent on the presence of glucose in the medium.
-
3.
Acetoacetate supported rapid respiration by AS-30D mitochondria in the presence of 0.1 mM malate.
-
4.
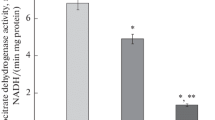
Succinly CoA acetoacetyl CoA transferase activity in AS-30D mitochondria was approximately 40 fold greater than that found in rat liver mitochondria.
-
5.
Addition of acetoacetate, but not β-hydroxybutyrate decreased conversion of [1-14C] acetate to14CO2, presumably by diluting the specific radioactivity of the acetyl CoA derived from the acetate tracer.
-
6.
In the presence of glucose, approximately one fourth of acetoacetate utilized was converted to lipid. This result is consistent with elevated lipogenesis postulated by the truncated TCA cycle hypothesis. These data demonstrate for the first time the flux of acetoacetate carbon to lipid and CO2 in hepatoma cells and suggest that increases in the ambient concentration of acetoacetate, occurring in fasting or malignant cachexia, could produce increases in the utilization of this ketone body by hepatoma cells containing 3-oxyacid CoA transferase activity.
Similar content being viewed by others
References
Smith DF, Walborg EF, Chang JP: Establishment of a transplantable ascites variant of a rat hepatoma induced by 3′-methyl-4-dimethylaminoacobenzene. Cancer Res 30: 2306–2309, 1970
Fiskum G: Mitochondrial respiration and calcium transport in tumor cells. In: G. Fiskum (ed). Mitochondrial physiology and pathology. Van Nostrand Reinhold, New York, 1986: 180–201
Nakashima RA, Paggi MG, Pedersen PL: Contributionsof glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Res 44: 5702–5706, 1984
Coleman PS, Lavietes BB: Membrane cholesterol, tumorigenesis and the biochemical phenotype of neoplasia. CRC Crit Rev Biochem 11: 341–393, 1981
Parlo RA, Coleman PS: Continuous pyruvate carbon flux to newly synthesized cholesterol and the suppressed evolution of pyruvate-generated CO2 in tumores: further evidence for a persistent truncated Krebs cycle in hepatomas. Biochim Biophys Acta 886: 169–176, 1986
Kelleher JK, Bryan BM, Mallet RT, Holleran AL, Murphy AN, Fiskum G: Analysis of tricarboxylic acid-cycle metabolism of hepatoma cells by comparison of14CO2 ratios. Biochem J 246: 633–639, 1987
Kovacevic Z, Popovic J, Brkljac O, Lelas S: Interation of metabolism of aspartate and inosine and energy state of malignant cells. Biochem J 247: 47–51, 1987
Dietzen DJ, Davis EJ: Oxidation of pyruvate, malate, citrate, and cytosolic reducing equivalents by AS-30D hepatoma mitochondria. Arch Biochem Biophys 305: 91–102, 1993
Fenselau A, Wallis K, Morris HP: Acetoacetate coenzyme a transferase activity in rat hepatomas. Cancer Res 35: 2315–2320, 1975
Fenselau A, Wallis K, Morris HP: Subcellular localization of aetoacetate coenzyme A transferase in rate hepatomas. Cancer Res 36: 4429–4433, 1976
Zhang WW, Lindahl R, Churchill P: Regulation of succinyl coenzymeA: acetoacetyl coenzyme A transferase in rat hepatoma cell lines. Cancer Res 50: 5858–5862, 1990
Sauer LA, Dauchy RT: Ketone body, glucose, lactic acid, and amino acid utilization by tumorsin vivo in fasted rats. Cancer Res 43: 3497–3503, 1983
Sauer LA, Nagel WO, Dauchy RT, Miceli LA, Austin JE: Stimulation of tumor growth in adult ratsin vivo during an acute fast. Cancer Res 46: 3469–3475, 1986
Sauer LA, Dauchy RT: Blood nutrient concentrations and tumor growthin vivo in rats: Relationships during the onset of an acute fast. Cancer Res 47: 1065–1068, 1987
Fiskum G, Pease A: Hydroperoxide-stimulated release of calcium from rat liver and AS-30D hepatoma mitochondria. Cancer Res 46: 3459–3463, 1986
Moreadith RW, Fiskum G: Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem 137: 360–367, 1984
Kelleher JK, Bryan III BM: A14CO2 Ratios Method for Detecting Pyruvate Carboxylation. Anal Biochem 151: 55–62, 1985
Kates M: Techniques of Lipidology. Laboratory Techniques in Biochemistry and Molecular Biology Vol. 3, part 2. 2nd ed. Elsevier, Amsterdam 1986
Melanby J, Williamson DH: Acetoacetate. In: H.U. Bergmeyer (ed). Methods of enzymatic analysis. 2nd ed. Vol 4, Academic Press, New York, pp 1840–1844
Ohe K, Morris HP, Weinhouse S: D-β-hydroxybutyrate dehydrogenase activity in liver and tumor. Cancer Res 27: 1360–1371, 1967
Zhang WW, Churchill S, Lindahl R, Churchill P: Regulation of D-beta-hydroxybutyrate dehydrogenase in rat hepatoma cell lines. Cancer Res 49: 2433–2437, 1989
Dietschy JM, Brown MS: Effect of alterations of the specific activity of the intracellular acetyl CoA pool on apparent rates of hepatic cholesterogenesis. J Lipid Res 15: 508–516, 1974
Moreadith RW, Lehninger AL: The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD (P) +-dependent malic enzyme. J Biol Chem 259: 6215–6221, 1984
Fearon KC, Borland W, Preston T, Tisdale MJ, Shenkin A, Calman KC: Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr 47: 42–48, 1988
Tisdale MJ: Role of acetoacetyl-CoA synthetase in acetoacetate utilization by tumor cells. Cancer Biochem Biophys 7: 101–107, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Briscoe, D.A., Fiskum, G., Holleran, A.L. et al. Acetoacetate metabolism in AS-30D hepatoma cells. Mol Cell Biochem 136, 131–137 (1994). https://doi.org/10.1007/BF00926073
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00926073