Abstract
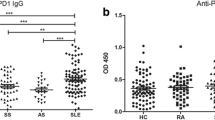
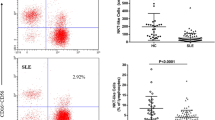
Hybridoma producing monoclonal antibody to proliferating cell nuclear antigen (PCNA/cyclin) (TOB7, IgG1 κ) was newly established. Using TOB7, PCNA was detected in the peripheral blood mononuclear cells (PBMC) in patients with systemic lupus erythematosus (SLE). Forty-four of 58 patients with SLE had PBMC expressing PCNA. The percentage of PCNA-positive PBMC in patients with SLE was 0–20% (mean: 2.63%) which was significantly higher (P<0.01) compared with normal controls (mean: 0.18%), patients with rheumatoid arthritis (mean: 0.83%), and patients with mixed connective tissue disease (mean: 0.38%). Patients with high numbers of PCNA positive PBMC tended to complicate pulmonary disorders (P<0.005), especially pulmonary fibrosis (P<0.005). In addition, the percentage of PCNA-positive cells in SLE patients correlated with the disease activity (r=0.45,P<0.01). The lymphocyte subsets of PCNA-positive PBMC were examined, and most of those cells belonged to CD4- or CD8-positive T-cell populations in three lupus patients. Our findings indicate that PCNA-positive activated PBMC are present in SLE patients and the percentage of PCNA-positive PBMC may be used as an indicator of disease activity.
Similar content being viewed by others
References
Miyachi K, Fritzler MJ, Tan EM: Autoantibody to a nuclear antigen in proliferating cells. J Immunol 121:2228–2234, 1978
Fritzler MJ, McCarty GA, Ryan JP, Kinsella TD: Clinical features of patients with antibodies directed against proliferating cell nuclear antigen. Arth Rheum 26:140–145, 1983
Ohata N: Clinical significance of antibodies to proliferating cell nuclear antigen. Rheumachi 27:79–87, 1987
Takasaki Y, Fishwild D, Tan EM: Characterization of proliferating cell nuclear antigen recognized by autoantibodies in lupus sera. J Exp Med 159:981–992, 1984
Ogata K, Ogata Y, Nakamura RM, Tan EM: Purification and N-terminal amino acid sequence of proliferating cell nuclear antigen (PCNA)/cyclin and development of ELISA for anti-PCNA antibodies. J Immunol 135:2623–2627, 1985
Takasaki Y, Deng JS, Tan EM: A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med 154:1899–1909, 1981
Mathews MB, Bernstein RM, Franza BR Jr, Garrels JI: Identity of the proliferating cell nuclear antigen and cyclin. Nature 309:374–376, 1984
Bravo R, Frank R, Blundell PA, Bravo HM: Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature 326:515–517, 1987
Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, Stillman B: Functional identity of proliferating cell nuclear antigen and a DNA polymerase-δ auxiliary protein. Nature 326:517–520, 1987
Smetana K, Gyorkey F, Chan PK, Tan EM: Proliferating cell nuclear antigen (PCNA) and human malignant tumor nuclear antigen (HMTNA) in nucleoi of human hematological malignancies. Blut 46:133–141, 1983
Takasaki Y, Robinson WA, Tan EM: Proliferating cell nuclear antigen in blast crisis cells of patients with chronic myeloid leukemia. J Natl Cancer Inst 73:655–661, 1984
Ogata K, Kurki P, Celis JE, Nakamura RM, Tan EM: Monoclonal antibody to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res 168:475–486, 1987
Kurki P, Ogata K, Tan EM: Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J Immnol Methods 109:49–59, 1988
Thaete LG, Ahnen DJ, Malkinson AM: Proliferating cell nuclear antigen (PCNA/Cyclin) immunocytochemistry as a labeling index in mouse lung tissues. Cell Tissue Res 256:167–173, 1989
Kurki P, Ogata K, Robinson CA, Williams GW, Tan EM: Proliferating cell nuclear antigen (PCNA) as a marker for a subset of activated human T-lymphocytes in blood and synovial fluid. Arth Rheum 29:586 (abstr B 24), 1986
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schallen JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. J Clin Invest 76:1865–1870, 1985
Ropes MW, Bennett EA, Cobb S, Jacox R, Jessary R: 1958 revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis 9:175–179, 1958
Lahita RG, Bradlow HL, Ginzler E, Pang S, New M: Low plasma androgens in women with systemic lupus erythematosus. Arth Rheum 30:241–248, 1987
Sharp GC, Irvin WS, Tan EM, Gould RG, Holman HR: Mixed connective tissue disease: An apparently distinct rheumatic disease syndrome associated with a specific antibody to extractable nuclear antigen (ENA). Am J Med 52:148–157, 1972
Oi V, Herzenberg LA:In Selected Methods in Cellular Immunology, BB Mishell, E Shigii (eds). San Francisco, WH Freeman, 1980, p 351
Boyum A: Separation of leukocytes from blood and bone marrow. Scand J Clin Lab Invest 21:97–105, 1968
Campbell DH, Garvey FS, Cremer NE, Sussdorf DH: Preparation of fluorescein-conjugated gamma globulin.In Methods in Immunology. New York, WA Benjamin, 1970, p 351
Farr RS: A quantitative immunochemical measure of the primary interaction between I*BSA and antibody. J Infect Dis 103:239–262, 1958
Northway JD, Tan EM: Differentiation of antinuclear antibodies giving speckled staining patterns in immunofluorescence. Clin Immunol 1:140–154, 1972
Lowry OH, Rosebrough NJ, Farr AL: Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275, 1951
Jasin HE, Ziff M: Immunoglobulin synthesis by peripheral blood cells in systemic lupus erythematosus. Arth Rheum 18:219–228, 1975
Letwin DA, Cohen PL, Winfield JB: Characterization of warm-reactive IgG anti-lymphocyte antibody in systemic lupus erythematosus. Relative specificity for mitogenactivated T cells and their soluble products. J Immunol 130:181–186, 1983
Fu SM, Chiroazzi N, Wang CY, Montaxeri G, Kunkel HG, Ko HS, Gottlieb AB: Ia bearing T lymphocytes in man: Their identification and role in the generation of allogenic helper activity. J Exp Med 148:1423–1428, 1978
Trowbridge IS, Omary MB: Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci USA 78:3039–3043, 1981
Uchiyama T, Broder S, Waldman TA: A monoclonal antibody (anti-Tac) reactive with activated and functional mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac cells. J Immunol 126:1393–1397, 1981
Boumpas DT, Tsokos GC, Mann DL, Eleftheriades EG, Harrs CC, Mark GE: Increased proto-oncogene expression in peripheral blood lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arth Rheum 29:755–760, 1986
Gerdes J, Schwab U, Lemke H, Stein H: Production of a mouse monoclonal antibody reactive with a nuclear antigen associated with cell proliferation. Int J Cancer 31:13–18, 1983
Haynes, BF, Hemler ME, Mann DL, Eisenbarth GS, Shelhamer J, Mostowski HS, Thomas CA, Strominger JL, Fauci AS: Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol 126:1409–1413, 1981
Yu DTY, Winchester RJ, Fu SM, Gibofsky A, Ko HS, Kunkel HG: Peripheral blood Ia-positive T cells. J Exp Med 151:91–100, 1980
Kurki P, Lotz M, Ogata K, Tan EM: Proliferating cell nuclear antigen (PCNA)/cyclin in activated human T lymphocytes. J Immunol 138:4114–4120, 1987
Volk HD, Kopp J, Korner IJ, Jahn S, Grunow R, Barthelmes H, Fiebig H: Correlation between the phenotype and the functional capacity of activated T cells in patients with active systemic lupus crythematosus. Scand J Immunol 24:109–114, 1986
Cotner T, Williams JM, Christenson L, Shapiro HM, Strom TB, Strominger J: Simultaneous flow cytemetric analysis of human T cell activation antigen expression and DNA content. J Exp Med 157:461–472, 1983
Blaese RM, Grayson J, Steinberg AD: Increased immunoglobulin-secreting cells in the blood of patients with active systemic lupus erythematosus. Am J Med 69:345–350, 1980
Hirano T: The appearance of Ia+T cells in systemic lupus erythematosus.In Cellular, Molecular and Genetic Approaches to Immunodiagnosis and Immunotherapy, K Kano (ed). Tokyo, University of Tokyo Press, 1987, pp 441–445
Carney WP, Iacovicello V, Hirsch MS: Functional properties of T lymphocytes and their subsets in cytomegalovirus mononucleosis. J Immunol 130:390–393, 1983
Crawford DH, Brickell, P, Tidman N, McConnell I, Hoffband AV, Janossy G: Increased numbers of cells with suppressor T cell phenotype in peripheral blood of patients with infectious mononucleosis. Clin Exp Immunol 43:291–297, 1981
Prelich G, Kostura M, Marshak DR, Mathews MB, Stillman B: The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature 326:471–475, 1987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murashima, A., Takasaki, Y., Ohgaki, M. et al. Activated peripheral blood mononuclear cells detected by murine monoclonal antibodies to proliferating cell nuclear antigen in active lupus patients. J Clin Immunol 10, 28–37 (1990). https://doi.org/10.1007/BF00917495
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00917495