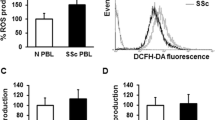
Abstract
Neutrophils from the synovial fluid (SFN) of 10 patients with active rheumatoid arthritis (RA) were investigated to determine the generation of oxygen intermediates (OI) (O −2 , H2O2, OH·), chemiluminescence, and lysosomal enzymes (lysozyme and β-glucuronidase). Lymphocytes from healthy individuals were cocultured at 37°C for 17 hr with SFN from the patients and the number of OKT4+, OKT8+, and OKT3+ cells and the response to mitogens were determined. A markedly increased OI and slightly elevated lysosomal enzyme levels were observed in SFN from patients. Coculture of lymphocytes with SFN resulted in a decreased number of OKT4+ and OKT8+ cells and a greatly reduced response to Con A and mildly diminished response to PHA, while OKT3+ cells were not affected. The simultaneous addition of superoxide dismutase and catalase restored the impairment of monoclonal antibody reaction and lymphocyte responsiveness almost to control levels. It is suggested that the disturbed immunoreactivity of synovial fluid lymphocytes from RA patients may be due to increased OI generated by stimulated neutrophils.
Similar content being viewed by others
References
Fridovich I: Oxygen radicals, hydrogen peroxide and oxygen toxicity.In Free Radicals and Inflammation, WA Pryor (ed). New York, Academic Press, 1976, pp 239–251
Salin ML, McCord JM: Free radicals and inflammation. Protection of phagocytosing leukocytes by superoxide dismutase. J Clin Invest 56:1319–1323, 1976
McCord JM, Fridovich I: The biology and pathology of oxygen radicals. Ann Intern Med 89:122–127, 1978
Sacks T, Moldow CF, Craddock PR, Bowers TK, Jacob HS: Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. J Clin Invest 62:1161–1167, 1978.
Klebanoff SJ: Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol 12:117–142, 1975
Boxer LA, Oliver JM, Spielberg SP, Allen JM, Schulman BA, Schulman JD: Protection of granulocytes by vitamin E in glutathione synthetase deficiency. N Engl J Med 301:901–905, 1979
Spielberg SP, Boxer LA, Corash LM: Improved erythrocyte survival with high-dose vitamin E chronic hemolyzing G6PD and glutathione synthetase deficiencies. Ann Intern Med 90:53–54, 1979
Necheles TF, Maldonado N, Barquet-Chediak H: Homozygous erythrocyte glutathione-peroxidase deficiency: Clinical and biochemical studies. Blood 33:164–169, 1969
Skosey JL, Chow DC, Musinow S, May J, Gestautas V, Niwa Y: Effect of oxygen tension on human peripheral blood leukocytes. J Cell Biol 88:358–363, 1981
Metzger Z, Hoffeld JT, Oppenheim JJ: Macrophage-mediated suppression. J Immunol 124:983–988, 1980
Nishida H, Tanimoto K, Akaoka H: Effect of free radicals on the lymphocytes. Clin Immunol Immunopathol 19:319–324, 1981
Murray HW, Cohn ZA: Macrophage oxygen-department antimicrobial activity. I. Susceptibility of toxoplasma gondii to oxygen intermediates. J Exp Med 150:938–949, 1979
Murray HW, Juangbhnich CW, Nathan CF, Cohn ZA: Macrophage oxygen-department antimicrobial activity. II. The role of oxygen intermediates. J Exp Med 150:950–964, 1979
Keystone EC, Schorlemmer HU, Pope C, Allison AC: Zymosan-induced arthritis. Arth Rheum 20:1394–1401, 1977
Gerbe DA: Low free serum histidine concentration in rheumatoid arthritis. J Clin Invest 55:1164–1173, 1975
Pinals RS, Harris ED, Burnett JB, Gerbe DA: Treatment of rheumatoid arthritis with L-histidine: A randomized, placebo-controlled, double-blind trial. J Rheumatol 4:414–419, 1977
Johnston RB Jr, Lehmeyer JE: Elaboration of topic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest 57:836–841, 1976
Biberfeld G, Nilsson E, Biberfeld P: T lymphocyte subpopulation in synovial fluid of patients with rheumatic disease. Arth Rheum 22:978–982, 1979
Burmester GR, Kalden JR, Peter HH, Schedel I, Beck P, Wittenborg A: Immunological and functional characteristics of peripheral blood and synovial fluid lymphocytes from patients with rheumatoid arthritis. Scand J Immunol 7:405–417, 1978
Abrahamsen TC, Froland SS, Natving JB: In vitro mitogen stimulation of synovial fluid lymphocytes from rheumatoid arthritis and juvenile rheumatoid arthritis patients: Dissociation between the response to antigens and polyclonal mitogens. Scand J Immunol 7:81–90, 1978
Van de Putte LBA, Meijer CJLM, Lafeber GJM, Kleinjan R, Cats A: Lymphocytes in rheumatoid and nonrheumatoid synovial fluids. Ann Rheum Dis 35:451–455, 1976
Vernon-Roberts B, Currey LHF, Perrin J: T and B cells in the blood and synovial fluid of rheumatoid patients. Ann Rheum Dis 33:430–434, 1974
Brenner AI, Scheinberg MA, Cathcart ES: Surface characteristics of synovial fluid and peripheral blood lymphocytes in inflammatory arthritis. Arth Rheum 18:297–303, 1975
Zembala M, Lemmel EM: Inhibitory factor(s) of lymphoproliferation produced by synovial fluid mononuclear cells from rheumatoid arthritis patients: The role of monocytes in suppression. J Immunol 125:1087–1092, 1980
Reinherz EL, Schlossman SF: Current concepts in immunology. Regulation of the immune response—Inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med 303:370–373, 1980
Reinherz EL, Kung PC, Goldstein G, Schlossman SF: Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci USA 76:4061–4065, 1979
Skosey JL, Chow DC, Damgaard E, Sorensen LB: Effect of cytocharasin B on the response of human polymorphonuclear leukocytes to zymosan. J Cell Biol 57:237–240, 1973
Cohen PP: Suspending media for normal tissues.In Monometric techniques and tissues metabolism, WW Umbreit, RH Burris, JF Stauffer (eds). Minneapolis, Burgess, 1957, pp 149–150
Massey V: The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta 34:255–256, 1959
Root RK, Metcalf JA: H2O2 release from human granulocytes during phagocytosis. J Clin Invest 60:1266–1279, 1977
Klebanoff SJ, Rosen H: Ethylene formation by polymorphonuclear leukocytes. J Exp Med 148:490–505, 1978
Allen RC, Loose LD: Phagocytic activation of a luminoldependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun 69:245–252, 1976
Baehner RL, Karnovsky MJ, Karnovsky ML: Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest 47:187–192, 1969
Wright DC, Malawista SE: The mobilization and extracellular release of granular enzymes from human leukocytes during phagocytosis. J Cell Biol 53:788–797, 1972
Bergmeyer HU, Bernt E, Hess B: Lactic dehydrogenase.In Methods of Enzymatic Analysis, Bergmeyer HU (ed). New York, Academic Press, 1963, pp 736–743
Lohrmann HP, Novikovs L, Graw RG: Stimulatory capacity of human T and B lymphocytes in the mixed leukocyte culture. Nature (Lond) 250:144–149, 1974
Skosey JL, Damgaard E, Chow DC, Sorensen LB: Modification of zymosan-induced release of lysosomal enzymes from polymorphonuclear leukocytes by cytochalasin B. J Cell Biol 62:625–634, 1974
Reinherz EL, Kung PC, Goldstein G, Schlossman SF: Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol 123:2894–2896, 1979
Stobo JD, Paul ER, Van Scoy S: Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest 57:319–328, 1976
Niwa Y, Kanoh T: Immunological behaviour following rubella infection. Clin Exp Immunol 37:470–476, 1979
Niwa Y, Ishimoto K, Yokoyama M: The impairment of lymphocytes influenced by stimulated neutrophils from the patients with systemic lupus erythematosus and rheumatic arthritis. J Allergy (Japanese) 29:981–991, 1980
Williams RC Jr, Debord JR, Mellbye OJ: Studies of T and B-lymphocytes in patients with connective tissue diseases. J Clin Invest 52:283–295, 1973
Froland SS, Natving JB, Husby G: Immunological characterization of lymphocytes in synovial fluid from patients with rheumatoid arthritis. Scand J Immunol 2:67–72, 1973
Reiss M, Roos D: Differences in oxygen metabolism of phagocytosing monocytes and neutrophils. J Clin Invest 61:480–488, 1978
Cochrane CG: Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol 9:97–162, 1968
Weissman G: Lysosomal mechanisms of tissue injury in arthritis. N Engl J Med 286:141–147, 1972
Janoff A: At least three human neutrophil lysosomal proteases are capable of degrading joint connective tissues. Ann NY Acad Sci 256:402–408, 1975
Cochrane CG, Unanue ER, Dixon FJ: A role of polymorphonuclear leukocytes and complement in nephrotoxic nephritis. J Exp Med 122:99–116, 1965
Goodwin JS, Webb DR: Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol 15:106–122, 1980
Kurland JI, Bockman R: Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med 147:952–958, 1978
Rice L, Laughter A, Twomey JJ: Three suppressor systems human blood that modulate lymphoproliferation. J Immunol 122:991–996, 1979
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Niwa, Y., Sakane, T., Shingu, M. et al. Effect of stimulated neutrophils from the synovial fluid of patients with rheumatoid arthritis on lymphocytes—A possible role of increased oxygen radicals generated by the neutrophils. J Clin Immunol 3, 228–240 (1983). https://doi.org/10.1007/BF00915347
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00915347