Abstract
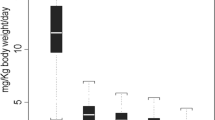
Cyclosporine G (OG 37-324) reportedly is an efficacious immunosuppressant with less nephrotoxicity than cyclosporine A. This is a prospective randomized double-blinded trial comparing cyclosporine G and cyclosporine A in cadaveric renal transplantation. Patient and graft survival, as well as major infectious complications, were not different between the two groups. Objective parameters of renal function, including serum creatinine, creatinine clearance, and inulin clearance, were routinely performed. These generally demonstrated less nephrotoxicity in those patients treated with cyclosporine G compared with cyclosporine A. Minor elevations of alanine aminotransferase were noted in the cyclosporine G-treated patients but this was not associated with acute morbidity. Overall, cyclosporine G appears to be equally as effective as cyclosporine A, but demonstrated notably less nephrotoxicity.
Similar content being viewed by others
References
The Canadian Multicentre Transplant Study Group (1986) A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med 314: 1219–1225
Najarian JS (1985) A single institution, randomized, prospective trial of cyclosporine versus azathioprine-antilymphocyte globulin for immunosuppression in renal allograft recipients. Ann Surg 201: 142–157
Henry ML, Sommer BG, Ferguson RM (1985) Beneficial effects of cyclosporine compared with azathioprine in cadaveric renal transplantation. Am J Surg 150: 533–536
Masri MA, Naiem M, Pingle S, Daar AS (1988) Cyclosporine A versus cyclosporine G: a comparative study of survival, hepatotoxicity, nephrotoxicity, and splenic atrophy in BALB/c mice. Transplant Int 1: 13–18
Rooth P, Dawidson I, Clothier N, Diller K (1988) In vivo fluorescence microscopy of kidney subcapsular blood flow in mice. Effects of cyclosporine, (Nva2)-cyclosporine, and isradipine, a new calcium antagonist. Transplantation 46: 566–569
Tejani A, Lancman I, Pomrantz A, Khawar M, Chen C (1988) Nephrotoxicity of cyclosporine A and cyclosporine G in a rat model. Transplantation 45: 184–187
Adams M, Bennett W, Danovitch G, Dawidson I, Leichtman A, Light J, Salomon D (1992) Preliminary evidence of the safety and efficacy of OG37-325, a cyclosporine analogue, in human renal transplantation (abstract). Am Soc Transplant Phys 11: 71
Sommer BG, Henry ML, Ferguson RM (1986) Sequential conventional immunotherapy with maintenance cyclosporine following renal transplantation. Transplant Proc 18 [Suppl 1]: 69–75
Huser B, Thiel G, Oberholzer M, Beveridge T, Bianchi L, Mihatsch MJ, Landmann J (1992) The efficacy and tolerability of cyclosporine G in human kidney transplant recipients. Transplantation 54: 65–69
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Henry, M.L., Elkhammas, E.A., Davies, E.A. et al. A clinical trial of cyclosporine G in cadaveric renal transplantation. Pediatr Nephrol 9 (Suppl 1), S49–S51 (1995). https://doi.org/10.1007/BF00867684
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00867684