Summary
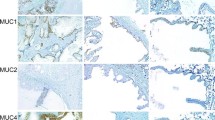
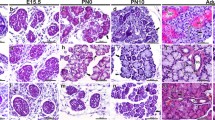
The expression and distribution of cytokeratins and vimentin in fifteen malignant salivary neoplasms were examined by immunocytochemical techniques using, five monoclonal antibodies (mAbs) against different epitopes of Cytokeratins (CKs) (mAbs PKK1, PKK2, and PKK3, identifying CKs 8, 18 and 19, CKs 7, 17 and 19, and CK 18, respectively) and Vimentin (mAbs V9 and V24). Antibody PKK1 gave strong reactions in all neoplasms showing the similarity of these tumours to other digestive system adenocarcinomas. Three general staining patterns of the neoplasms were recognized with respect to the reactivity of mAbs PKK2, PKK3, and V9. Mucoepidermoid cancer, salivary duct carcinoma and a clear cell carcinoma had a higher relative content of CKs 7, 17 and 19 than of CK 18. Adenoid cystic carcinoma showed the same CK pattern but in the periphery of the tumour cords vimentin was readily detected. In two acinic cell carcinomas, the relative content of CK 18 was higher than that of CKs 7, 17 and 19. Furthermore vimentin was expressed in the tumour cells. However, one mucoepidermoid carcinoma showed vimentin expression and two acinic cell carcinomas were vimentin negative and more reative for PKK2 than PKK3. Pecularities in CK expression were seen: squamous areas of mucoepidermoid carcinomas were stained by mAb PKK3 although CK 18 is not present in normal squamous epithelia or in squamous cell carcinomas of tongue and skin. In conclusion, the different salivary neoplasms can be distinguished on basis of IFP content. Such a differentiation fits with current theories of histogenesis, i.e. vimentin is seen in tumours presumed to arise from intercalated duct reserve cells, whilst the vimentin negative neoplasms would be expected to arise in excretory duct reserve cells.
Similar content being viewed by others
References
Batsakis JG (1979) Tumours of the head and neck. 2nd ed., Williams and Wilkins, Baltimore
Batsakis JG, Pinkston GR, Luna MA, Byers RM, Sciubba JJ, Tillery GW (1983) Adenocarcinomas of the oral cavity. A clinoco-pathologic study of terminal duct carcinomas. J Laryngol Otol 97:825–835
Batsakis JG (1983) The pathology of head and neck tumours. The myopithelial cell and its participation in salivary gland neoplasms. Head Neck Surg 5:222–233
Bienengräber V (1978) Ultrastruktur und klinikopathologische Aspekte des Mucoepidermoid Tumors der Mundspeicheldrüsen. Arch Geschwulstforsch 48:35–49
Caselitz J, Osborn M, Seifert G, Weber K (1981) Intermediate sized filament proteins (prekeratin, vimentin, desmin) in the normal parotid gland and parotid gland tumours. Immunofluorescence study. Virchows Arch A (Pathol Anat) 393:273–286
Caselitz J, Osborn M, Wustrow J, Seifert G, Weber K (1982) The expression of different intermediate filaments in human salivary glands and their tumours. Pathol Res Pract 175:266–278
Caselitz J, Becker J, Seifert G, Weber K, Osborn M (1984) Co-expression of keratin and vimentin filaments in adenoid cystic carcinoma of salivary glands. Virchows Arch A (Pathol Anat) 403:337–344
Caselitz J, Osborn M, Hamper K, Wustrow J, Rauchfuss A, Weber K (1986) Pleomorphic adenomas, adenoidcystic carcinomas and adenolymphomas of salivary glands analysed by a monoclonal antibody against myoepithelial cells. An immunohistochemical study. Virchows Arch A (Pathol Aanat) 409:805–816
Cooper D, Schermer A, Sun TT (1985) Biology of disease. Classification of human epithelial and their neoplasms using monoclonal antibodies to keratins: Strategies, applications and limitations. Lab Invest 52:243–256
Dardick I, Kahn H, van Nostrand P, Baumal R (1984) Salivary gland monomorphic adenoma. Ultrastructural, Immunoperoxidase and histogenetic aspects. Am J Pathol 115:334–348
Erlandson RA, Cardon-Cardo C, Higgins PJ (1984) Histogenesis of benign pleomorphic adenoma (mixed tumour) of the major salivary glands, and ultrastructural and immunohistochemical study. Am J Surg Pathol 8:803–820
Geiger S, Geiger B, Leitner O, Marshak G (1987) Cytokeratin polypeptides expression in different epithelial elements of human salivary glands. Virchows Arch A 410:403–414
Gould VE (1986) Current topics. Histogenesis and differentiation. A revaluation of these concepts as criteria for the classification of tumours. Human Pathol 17:211–215
Gustafsson H, Kjörell V, Carlsöö B (1985) Cytoskeletal Proteins in onkocytic tumours of the parotial gland. Arch Otolaryngol 111:99–105
Gustafsson H, Carlsöö B, Kjörell U, Thornell L-E (1986a) Ultrastructural and immunohistochemical aspects of carcinoma in mixed tumours. Am J Otolaryngol 7:218–230
Gustafsson H, Carlsöö B, Kjörell U, Thornell L-E (1986b) Immunohistochemical and ultrastructural observations on adenoidcystic carcinoma of salivary glands. A study with special reference to intermediate filaments and proteoglycan particles. Acta Otolaryngol 102:152–160
Gustafsson H, Kjörell U, Eriksson A, Virtanen I, Thornell L-E (1988) Distribution of intermediate filament proteins in developing and adult salivary glands in man. Anatomy and Embryology (in press)
Hamperl H (1970) The myothelia (Myoepithelial cells) Normal state, regressive changes, hyperplasia, tumours. Curt Top Pathol 53:161–220
Herman CJ, Moesker O, Kant A, Huysmans A, Voijs GP, Ramaekers FCS (1983) Is renal (Grawitz) tumour a carcinosarcoma? Evidence form analysis of intermediate filament types. Virchows Arch B (Cell Pathol) 44:73–83
Holthöfer H, Miettinen M, Letho VP, Lindner E, Alfthan OA, Virtanen I (1983) Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney specific antibodies, anti-intermediate filaments and lecitins. Lab Invest 49:317–326
Hübner G, Klein HJ, Kleinsasser O, Schieffer HG (1971) Role of myoepithelial cells in the development of salivary gland tumours. Cancer 27:1255–1261
Kahn HJ, Baumal R, Marks A, Dardick I, van Nostrand AWP (1985) Myoepithelial cells in salivary gland tumours. An immunohistochemical study. Arch Pathol Lab Med 109:190–195
Kjörell U, Thornell L-E, Lehto V-P, Virtanen I, Whalen RG (1987) A comparative analysis of intermediate filament proteins in bovine heart Purkinje fibers. Eur J Cell Biol 44:68–78
Krepler R, Denk H, Artlieb V, Moll R (1982) Immunohistochemistry of intermediate filament proteins present in pleomorphic adenomas of the human parotial gland. Characterization of different cell types in the same tumour. Differentiation 21:191–199
Mc Nutt MA, Bolen JW, Gown AM, Hammar SP, Vogel AM (1985) Co-expression of intermediate filaments in human epithelial neoplasms. Ultrastruct Pathol 9:31–43
Miettinen M, Lehto V-P, Virtanen I (1984a) Antibodies to intermediate filament proteins in the diagnosis and classification of human tumours. Ultrastruct Pathol 7:83–107
Miettinen M, Franssila K, Lehto VP, Paasivou R, Virtanen I (1984b) Expression of intermediate filament proteins in thyroid gland and thyroid tumours. Lab Invest 60:262–269
Miettinen M, Clark R, Lehto V-P, Virtanen I, Dajmanov I (1985a) Intermediate filament proteins in parathyroid glands and parathyroid adenomas. Arch Pathol Lab Med 109:986–989
Miettinen M, Letho V-P, Dahl D, Virtanen I (1985b) Varying expression of cytokeratin and neurofilaments in neuroendocrine tumours of human gastrointestinal tract. Lab Ivest 52:429–436
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumours and cultured cells. Cell 31:11–24
Nicolatou O, Harwick RD, Putong P, Leifer C (1979) Ultrastructural characterization of intermediate cells of mucoepidermoid carcinoma of the parotis. Oral Surg 48:324–336
Osborn M, Weber K (1983) Biology of disease. Tumour diagnosis by intermediate filament typing: A novel tool for surgical pathology. Lab Invest 48:372–394
Osborn M (1984) Components of the cellular cytoskeleton: A new generation of markers of histogenetic origin? J Invest Dermatol 82:443–445
Palmer RM, Lucas RB, Knight J (1985) Immunocytochemical identification of cell types in pleomorphic adenoma with particular reference to myoepithelial cells. J Pathol 146:213–220
Quinlan RA, Schiller DL, Hatzfeld M, Achtstätter T, Moll R, Jorcano JL, Magin TM, Franke WW (1985) Patterns of expression and organisation of cytokeratin intermediate filaments. Ann NY Acad Sci 455:282–306
Ramaekers FCS, Moesker O, Huysmans A, Schaart G, Westerhof G, Wagenaar S, Herman CJ, Voijs GP (1985) Intermediate filament proteins in the study of tumour heterogeneity; An in-depth study of tumours of the urinary and respiratory trackts. Ann NY Acad Sci 455:614–634
Rice RH, Green H (1979) Presence in human epidermal cells of a soluble protein precursor of the cross linked envelope: Activation of the cross linking by calcium ions. Cell 18:681–694
Saku T, Okabe H, Yagi Y, Sato E, Tsuda N (1984) A comparative study on the ummunolocalization of keratin and myosin in salivary gland tumours. Acta Pathol Jpn 34:1031–1040
Thackray AC, Sobin LH (1972) Histological typing of salivary gland tumours. International histological classification of tumours. No 7, Geneva, WHO
Virtanen I, Miettinen M, Lehto V-P (1985) Diagnostic application of monoclonal antibodies to intermediate filaments. Ann NY Acad Sci 455:635–648
Virtanen I, Kallajoki M, Närvänen O, Paranko J, Thornell L-E, Mietlinen M, Lehto V-P (1986a) Peritubular myoid cells of human rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec 215:10–20
Virtanen I, Kariniemi A-L, Holthöfer H (1986b) Fluorochrome-coupled lectins reveal distinct cellular domains in human epidermis. J Histochem Cytochem 34:307–315
Warner T, Seo IS, Azen EA, Hafez GR, Zarling TA (1985) Immunocytochemistry of acinic cell carcinomas and mixed tumours of salivary glands. Cancer 56:2221–2227
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gustafsson, H., Virtanen, I. & Thornell, LE. Expression of cytokeratins and vimentin in salivary gland carcinomas as revealed with monoclonal antibodies. Vichows Archiv A Pathol Anat 412, 515–524 (1988). https://doi.org/10.1007/BF00844287
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00844287