Summary
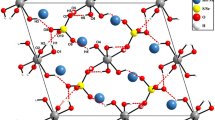
The crystal structures of the new, hydrothermally synthesized, isotypic compounds Co3(SeO3)3·H2O and Ni3(SeO3)3·H2O were determined by direct and Fourier methods and refined toR w=0.023, 0.032 using single crystal X-ray data up to sinϑ/λ=0.81 Å−1 [space group P\(\bar 1\),a=8.102 (2), 7.986 (3) Å;b=8.219 (2), 8.133 (3) Å;c=8.572 (2), 8.422 (3) Å, α=69.15 (1), 69.50 (1)°; β=62.88 (1), 62.50 (1)°; γ=67.23 (1), 67.64 (1)°;Z=2]. The structures are built up from [Me 5(SeO3)6·2H2O]2− sheets containing three crystallographically different types of octahedrally coordinatedMe 2+ and trigonal pyramidal coordinated Se4+ atoms, respectively. These sheets are linked only by a fourth type ofMe 2+[6] atom. All coordination polyhedra deviate significantly from their ideal shapes, bond lengths within the extremly distortedMe(4)O6 polyhedra range from 1.983 (2) Å to 2.403 (2) Å in Co3(SeO3)3·H2O and from 1.987 (4) Å to 2.301 (3) Å in the Ni compound, O-Se-O bond angles were found between 92.8 (2)° and 104.9 (1)°. Hydrogen bond lengths are 2.802 (3)Å and 2.600 (4)Å in the Co compound, and 2.762 (6) Å and 2.561 (6) Å in Ni3(SeO3)3·H2O. The latter is one of the shortest known hydrogen bonds donated by a water molecule.
Zusammenfassung
Die Kristallstrukturen der neuen, hydrothermal synthetisierten, isotypen Verbindungen Co3(SeO3)3·H2O und Ni3(SeO3)3·H2O wurden mit direkten und Fourier-Methoden bestimmt und unter Verwendung von Einkristallröntgendaten bis sinϑ/λ=0.81 Å−1 aufR w-Werte von 0.023, 0.032 verfeinert [Raumgruppe P\(\bar 1\),a=8.102 (2), 7.986 (3) Å;b=8.219 (2), 8.133 (3) Å;c=8.572 (2), 8.422 (3) Å, α=69.15 (1), 69.50 (1)°; β=62.88 (1), 62.50 (1)°; γ=67.23 (1), 67.64 (1)°;Z=2]. Die Strukturen werden von [Me 5(SeO3)6·2H2O]2− Schichten aufgebaut, die je drei kristallographisch unterschiedliche Arten von oktaedrisch koordiniertenMe 2+ und trigonal pyramidal koordinierten Se4+ Atomen enthalten. Diese Schichten sind nur durch eine vierte Art vonMe 2+[6] Atomen verknüpft. Alle Koordinationspolyeder weichen deutlich von ihren Idealformen ab, Bindungslängen in den extrem verzerrtenMe(4)O6 Polyedern variieren zwischen 1.983 (2) Å und 2.403 (2) Å in Co3(SeO3)3·H2O und zwischen 1.987 (4) Å und 2.301 (3) Å in der Ni-Verbindung, O-Se-O-Bindungswinkel liegen zwischen 92.8 (2)° und 104.9 (1)°. Wasserstoffbrückenlängen sind 2.802 (3) Å und 2.600 (4) Å in der Co-Verbindung, und 2.762 (6) Å und 2.561 (6) Å in Ni3(SeO3)3·H2O. Letztere ist eine der kürzesten bekannten Wasserstoffbrücken eines Wassermoleküls.
Similar content being viewed by others
References
Wildner M. (1988) Diploma Thesis. Univ. Wien
Wildner M. (1988) Z. Krist.185: 499
Wildner M. (1990) N. Jb. Miner. Mh.1990: 353
Ibers J. A., Hamilton W. C. (eds.) (1974) International Tables for X-Ray Crystallography, Vol. IV. Kynoch Press, Birmingham
Zachariasen W. H. (1967) Acta Cryst.23: 558
Brown I. D. (1981) Structure and Bonding in Crystals, Vol. II-14. Academic Press, New York
Brown I. D., Shannon R. D. (1973) Acta Cryst.A29: 266
Robinson K., Gibbs G. V., Ribbe P. H. (1971) Science172: 567
Fischer R., Zemann J. (1974) Handbook of Geochemistry II-3, 34-A. Springer, Berlin Heidelberg New York
Giester G. (1989) Monatsh. Chem.120: 661
Effenberger H., Pertlik F. (1984) Z. Krist.166: 129
Effenberger H. (in preparation) Monatsh. Chem.
Krishnamachari N., Calvo C. (1972) Acta Cryst.B28: 2883
Tordjman I., Guitel J. C., Durif A., Averbuch M. T., Masse R. (1978) Mat. Res. Bul.13: 983
Keller P., Riffel H., Zettler F., Hess H. (1981) Z. Anorg. Allg. Chem.474: 123
Jasper-Tönnies B., Müller-Buschbaum H. (1984) Z. Anorg. Allg. Chem.517: 161
Ruszala F. A., Anderson J. B., Kostiner E. (1977) Inorg. Chem.16: 2417
Sasaki S., Takéuchi Y. (1982) Z. Krist.158: 279
Buckley A. M., Bramwell S. T., Day P. (1990) J. Sol. State Chem.86: 1
Chiari G., Ferraris G. (1982) Acta Cryst.B38: 2331
Wildner M. (1990) Thesis. Univ. Wien
Dowty E. (1989) Atoms. A Computer Program for Displaying Atomic Structures. Shape Software, Kingsport
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wildner, M. Crystal structures of Co3(SeO3)3·H2O and Ni3(SeO3)3·H2O, two new isotypic compounds. Monatsh Chem 122, 585–594 (1991). https://doi.org/10.1007/BF00811457
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00811457