Summary
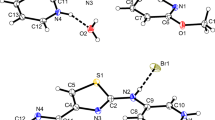
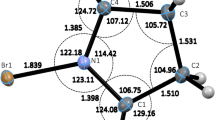
From a survey of spectroscopic and structural data of six corresponding 2-hydroxybenzamides and 2-hydroxythiobenzamides (amide, N-methylamide, N,N-dimethylamide, piperidide, morpholide, 2,6-dimethylpiperidide) remarkable similarities between O(N)-H ... O and O(N)-H ... S hydrogen-bonds are obtained, concerning both, hydrogen-bond patterns and hydrogen-bond strengths. In dilute solution the OH groups of all compounds are intramolecularly associated to the (thio)carbonyl O (S) atoms with distinctly larger hydrogen-bond strengths for primary and secondary amides [\(\bar v\)(O-H)=2950−3020 cm−1, δ(OH)=12.16−11.99 ppm] and thioamides [\(\bar v\)(O-H)=2960−3000 cm−1, δ(OH)=11.65−11.13 ppm], than for tertiary amides [\(\bar v\)(O-H)=3200−3250 cm−1, δ(OH)=9.95−8.95 ppm] and thioamides [\(\bar v\)(O-H)=3245−3330 cm−1, δ(OH)=8.09−7.06 ppm]. In the solid state, the OH groups of the primary and secondary (thio)amides are also engaged in rather strong intramolecular O-H ... O=C [O ... O=2.51 Å,\(\bar v\)(O-H)=2700−2750 cm−1] and O-H ... S=C [O ... S=2.90−2.94 Å,\(\bar v\)(O-H)=2700−2840 cm−1] hydrogen-bonds; thetrans-NH groups of the primary (thio)amides and the NH groups of the secondary (thio)amides connect the molecules to N-H ... O-H [N ... O=2.93−3.10 Å,\(\bar v\)(N-H)=3319−3407 cm−1] hydrogen-bonded chains; the remainingcis-NH groups of the primary (thio)amides give rise to eight-membered cyclic dimers via N-H ... O=C [N ... O=2.93 Å,\(\bar v\)(N-H)=3226 cm−1] and N-H ... S=C [N ... S=3.46−3.47 Å,\(\bar v\)(N-H)=3233−3277 cm−1] hydrogen-bonds. Contrary, the OH groups of the tertiary (thio)amides are intermolecular associated in the solid state and link the molecules to O-H ... O=C [O ... O=2.63−2.75 Å,\(\bar v\)(O-H)=3075−3135 cm−1] and O-H ... S=C [O ... S=3.18−3.26 Å,\(\bar v\)(O-H)=3130−3190 cm−1] hydrogen-bonded chains.
Zusammenfassung
Aus einer Zusammenstellung von spektroskopischen und strukturellen Daten von sechs entsprechenden 2-Hydroxybenzamiden und 2-Hydroxythiobenzamiden (Amid, N-Methylamid, N,N-Dimethylamid, Piperidid, Morpholid, 2,6-Dimethylpiperidid) ergeben sich bemerkenswerte Analogien zwischen O(N)-H ... O und O(N)-H ... S H-Brücken, die sowohl die H-Brücken-Muster als auch die H-Brücken-Stärken betreffen. In verdünnter Lösung sind die OH-Gruppen aller Verbindungen intramolekular mit den O(S)-Atomen der (Thio)Carbonylgruppen assoziiert, wobei die H-Brücken bei den primären und sekundären Amiden [\(\bar v\)(O-H)=2950−3020 cm−1, δ(OH)=12.16−11.99 ppm] und Thioamiden [\(\bar v\)(O-H)=2960−3060 cm−1, δ(OH)=11.65−11.13 ppm] deutlich stärker sind, als bei den tertiären Amiden [\(\bar v\)(O-H)=3200−3250 cm−1, δ(OH)=9.95−8.95 ppm] und Thioamiden [\(\bar v\)(O-H)=3245−3330 cm−1, δ(OH)=8.09−7.06 ppm]. Im Festkörper weisen die primären und sekundären (Thio)Amide ebenfalls sehr starke intramolekulare O-H ... O=C [O ... O=2.51 Å,\(\bar v\)(O-H)=2700−2750 cm−1] und O-H ... S=C [O ... S=2.90−2.94 Å,\(\bar v\)(O-H)=2700−2840 cm−1] H-Brücken auf; dietrans-NH-Gruppen der primären (Thio)Amide und die NH-Gruppen der sekundären (Thio)Amide verknüpfen die Moleküle über N-H ... O-H H-Brücken [N ... O=2.93−3.10 Å,\(\bar v\)(N-H)=3318−3407 cm−1] zu Ketten; die verbleibendencis-NH-Gruppen der primären (Thio)Amide bilden zyklische, über N-H ... O=C [N ... O=2.93 Å,\(\bar v\)(N-H)=3226 cm−1] und N-H ... S=C [N ... S=3.46−3.47 Å,\(\bar v\)(N-H)=3233−3277 cm−1] H-Brücken gebundene, 8-Ring-Dimere. Im Gegensatz dazu sind die OH-Gruppen der tertiären (Thio)Amide im Festkörper intermolekular assoziiert und verknüpfen die Moleküle über O-H ... O=C [O ... O=2.63−2.75 Å,\(\bar v\)(O-H)=3075−3135 cm−1] und O-H ... S=C [O ... S=3.18−3.26 Å,\(\bar v\)(O-H)=3130−3190 cm−1] H-Brücken zu Ketten.
Similar content being viewed by others
References
Derkosch J., Mikenda W., Steinwender E. (1987) Spectrochim. Acta43 A: 823
Fong C. W., Grant H. G. (1981) Aust. J. Chem.34: 957
Pertlik F. (1990) Monatsh. Chem.121: 129
Pertlik F. (to be published)
Pertlik F. (1987) Monatsh. Chem.118: 1349
Van Roey P., Kerr K. A. (1981) Acta Cryst.B37: 1679
Kerr K. A., Van Roey P. (1979) Acta Cryst.B35: 2727
Kondo M. (1972) Bull. Chem. Soc. Japan45: 2710
Shome S. C., Mazumdar M. (1969) Anal. Chim. Acta46: 155
Berg U. (1976) Acta Chem. Scand.B30: 695
Banerjee K., Raychaudaury S. (1982) Bull. Chem. Soc. Japan55: 3621
Kondo M. (1979) Bull. Chem. Soc. Japan52: 521
Steinwender E., Mikenda W. (to be published)
Walter W. (1970) Z. Chem.10: 371
Ginzburg I. M., Bessonova N. N. (1975) Zh. Obshch. Khim.45: 622
Taylor R., Kennard O. (1983) Acta Cryst.B39: 133
Blake C. C. F., Small R. W. H. (1972) Acta Cryst.B28: 2201
Leiserowitz L., Tuval M. (1978) Acta Cryst.B34: 1230
Colleter J. C., Gadret M. (1967) Bull. Soc. Chim. France9: 3463
Etter M. C. (1982) J. Am. Chem. Soc.104: 1095
Etter M. C., Urbanczyk-Lipkowska Z., Ameli T. M., Panunto T. W. (1988) J. Cryst. Spectrosc. Res.18: 491
Lautie A., Froment F., Novak A. (1976) Spectrosc. Lett.9: 289
Shibaev R. P., Atovmyan L. O. (1968) Zh. Strukt. Khim.9: 90
Arte E., Feneau-Dupont J., Declercq J. P., Germain G., Van Meersche M. (1977) Cryst. Struct. Comm.6: 767
Walter W., Harto S., Voss J. (1976) Acta Cryst.B32: 2876
Kerr K. A., Van Roey P. (1979) Acta Cryst.B35: 2344
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steinwender, E., Mikenda, W. O-H ... O(S) hydrogen bonds in 2-hydroxy(thio)benzamides. Survey of spectroscopic and structural data. Monatsh Chem 121, 809–820 (1990). https://doi.org/10.1007/BF00808374
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00808374