Abstract
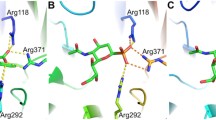
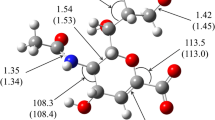
The inhibition of sialidase activity from influenza viruses A and B, parainfluenza 2 virus,Vibrio cholerae, Arthrobacter ureafaciens, Clostridium perfringens, and sheep liver by a range of 2-deoxy-2,3-didehydro-N-acetylneuraminic acid analogues modified at the C-4 position has been studied. All substitutions tested resulted in a decrease in the degree of inhibition of the bacterial and mammalian sialidases. For sialidases from influenza viruses A and B, on the other hand, most of the substitutions tested either had no significant effect on binding or, in the case of the basic amino and guanidino substituents, resulted in significantly stronger inhibition. The results for parainfluenza 2 virus sialidase were mostly intermediate, in that inhibition was neither significantly increased nor decreased by most of the modifications. We conclude that only the influenza A and B sialidase active sites possess acid groups correctly positioned to participate in charge-charge interactions in the region of C-4 of bound substrate, and that the C-4 binding pockets of the bacterial and mammalian sialidases examined are considerably smaller than is observed for either the influenza virus or parainfluenza virus sialidases.
Similar content being viewed by others
References
Drzeniek R (1972)Curr Top Microbiol Immunol 59:35–74.
Schauer R (1985)Trends Biochem Sci 10:357–60.
Klenk H-D, Compans RW, Choppin PW (1970)Virology 42:1158–62.
Palese P, Tobita K, Ueda M, Compans RW (1974)Virology 61:397–410.
Klenk H-D, Rott R (1988)Adv Virus Res 34:247–80.
Ashwell G, Morell AG (1974)Adv Enzymol 41:99–128.
Varghese JN, Laver WG, Colman PM (1983)Nature 303:35–40.
Varghese JN, McKimm-Breschkin J, Caldwell JB, Kortt AA, Colman PM (1992)Proteins Struct Funct Genet 14:327–32.
Chong AKJ, Pegg MS, Taylor NJ, von Itzstein M (1992)Eur J Biochem 207:335–43.
Chong AKJ, Pegg MS, von Itzstein M (1991)Biochem Int 24:165–71.
von Itzstein M, Wu W-Y, Kok G, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW, Colman PM, Ryan DM, Woods LM, Bethell RC, Hotham VJ, Cameron JM, Penn CR, submitted for publication.
von Itzstein M, Wu W-Y, Van Phan, T, Danylec B, Jin B (1991) International Patent App. WO9116320-A91.10.31 (9147) CA117:49151y.
Schreiner E, Zbiral E, Kleineidam RG, Schauer R (1991)Liebigs Ann Chem 129–34.
Zbiral E, Brandstetter HH, Christian R, Schauer R (1987)Liebigs Ann Chem 781–86.
Glänzer BI, Györgydeák Z, Bernet B, Vasella A (1991)Helvetica Chim Acta 74:343–69.
Meindl P, Bodo G, Palese P, Schulman J, Tuppy H (1974)Virology 58:457–63.
Cartwright EC, Pegg MS, Stewart WP, von Itzstein M, Colman PM (1989)Proc Aust Biochem Soc 21:P86.
Potier M, Mameli L, Belisle M, Dallaire L, Melancon SB (1979)Anal Biochem 94:287–96.
Chong AKJ, Pegg MS, von Itzstein M (1991)Biochim Biophys Acta 1077:65–71.
Myers RW, Lee RT, Lee YC, Thomas GH, Reynolds LW, Uchida Y (1980)Anal Biochem 101:166–74.
Segal IH (1975) InEnzyme Kinetics, pp. 210–12. New York: Wiley.
Okamoto K, Kondo T, Goto T (1987)Bull Chem Soc Jpn 60:631–36.
Burnet FM, Stone JO (1947)Aust J Exp Biol Med Sci 25:227–33.
Author information
Authors and Affiliations
Additional information
This paper is dedicated to the memory of Professor Dr E. Zbiral.
Rights and permissions
About this article
Cite this article
Holzer, C.T., Von Itzstein, M., Jin, B. et al. Inhibition of sialidases from viral, bacterial and mammalian sources by analogues of 2-deoxy-2,3-didehydro-N-acetylneuraminic acid modified at the C-4 position. Glycoconjugate J 10, 40–44 (1993). https://doi.org/10.1007/BF00731185
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00731185