Abstract
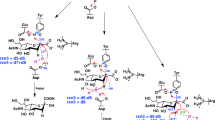
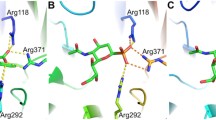
Streptococcus pneumoniae is a Gram-positive human pathogenic bacterium, which is the main cause of pneumonia and meningitis in children and the elderly. Three sialidases (or neuraminidases) encoded from Streptococcus pneumoniae could catalyze the cleavage of sialic acid linkages. This mechanism is directly connected with infection, apoptosis, and signaling, and usually considered to be one of the critical virulence factors. Type C neuraminidase (NanC) is unique because its primary product of Neu5Ac2en is considered to be an inhibitor to the other two sialidases. Experimentally, there are two different pathways for the formation mechanism of Neu5Ac2en catalyzed by NanC. In this work, a combined quantum mechanical and molecular mechanical approach was employed in all calculations. Starting from the covalent sialylated intermediate, we first examined the reaction to Neu5Ac2en and found the reaction prefers a direct proton abstraction mechanism rather than the water mediated proton abstraction mechanism. Free energy profiles can confirm that Neu5Ac2en is the major product of NanC. Functional roles of some important residues were also investigated, e.g., D315 acts as the proton acceptor during the formation of Neu5Ac2en, while the general base for the hydrolytic reaction to Neu5Ac. This study can facilitate the understanding of the catalytic mechanism of NanC and has the potential to aid in future inhibitor design studies.













Similar content being viewed by others
References
Kiefel MJ, von Itzstein M (2002) Chem Rev 102:471
Taylor G (1996) Curr Opin Struct Biol 6:830
Li N, Ren A, Wang X, Fan X, Zhao Y, Gao GF, Cleary P, Wang B (2015) Proc Natl Acad Sci U S A 112:238
King SJ (2010) Mol Oral Microbiol 25:15
King SJ, Hippe KR, Weiser JN (2006) Mol Microbiol 59:961
Bridy-Pappas AE, Margolis MB, Center KJ, Isaacman DJ (2005) Pharmacotherapy 25:1193
Manco S, Hernon F, Yesilkaya H, Paton JC, Andrew PW, Kadioglu A (2006) Infect Immun 74:4014
Parker D, Soong G, Planet P, Brower J, Ratner AJ, Prince A (2009) Infect Immun 77:3722
Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F (2006) Infect Immun 74:3360
von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Phan TV, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR (1993) Nature 363:418
Taylor NR, von Itzstein M (1994) J Med Chem 37:616
Chokhawala HA, Yu H, Chen X (2007) ChemBioChem 8:194
Mitchell FL, Neres J, Ramraj A, Raju RK, Hillier IH, Vincent MA, Bryce RA (2013) Biochem 52:3740
Pierdominici-Sottile G, Horenstein NA, Roitberg AE (2011) Biochem 50:10150
Frasch ACC (2000) Parasitol Today 16:282
Watts AG, Damager I, Amaya ML, Buschiazzo A, Alzari P, Frasch AC, Withers SG (2003) J Am Chem Soc 125:7532
Rogers IL, Naidoo KJ (2016) ACS Catal 6:6384
Gut H, King SJ, Walsh MA (2008) FEBS Lett 582:3348
Xu G, Li X, Andrew PW, Taylor GL (2008) Acta Crystallogr Sect F Struct Biol Cryst Commun 64:772
Xu G, Potter JA, Russell RJM, Oggioni MR, Andrew PW, Taylor GL (2008) J Mol Biol 384:436
Xu G, Kiefel MJ, Wilson JC, Andrew PW, Oggioni MR, Taylor GL (2011) J Am Chem Soc 133:1718
Owen CD, Lukacik P, Potter JA, Sleator O, Taylor GL, Walsh MA (2015) J Biol Chem 290:27736
Newstead SL, Potter JA, Wilson JC, Xu G, Chien C-H, Watts AG, Withers SG, Taylor GL (2008) J Biol Chem 283:9080
Warshel A, Levitt M (1976) J Mol Biol 103:227
Elstner M, Porezag D, Jungnickel G, Elsner J, Haugk M, Frauenheim T, Suhai S, Seigert G (1998) Phys Rev B58:7260
Elstner M, Frauenheim T, Kaxiras E, Seifert G, Suhai S (2000) Phys Status Solidi B217:357
Elstner M (2006) Theor Chem Accounts 116:316
Elstner M, Cui Q, Munih P, Kaxiras E, Frauenheim T, Karplus M (2003) J Comput Chem 24:565
Niehaus TA, Elstner M, Frauenheim T, Suhai S (2001) J Mol Struct (THEOCHEM) 541:185
Cui Q, Elstner M, Kaxiras E, Frauenheim T, Karplus M (2001) J Phys Chem B 105:569
MacKerell Jr AD, Bashford D, Bellott M, Dunbrack Jr RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher III WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1998) J Phys Chem B 102:3586
Field MJ, Bash PA, Karplus M (1990) J Comput Chem 11:700
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) J Chem Phys 79:926
Brooks III CL, Karplus M (1983) J Chem Phys 79:6312
Brooks III CL, Brunger A, Karplus M (1985) Biopoly 24:843
Ryckaert J-P, Ciccotti G, Berendsen HJC (1977) J Comput Phys 23:327
Steinbach PJ, Brooks BR (1994) J Comput Chem 15:667
Torrie GM, Valleau JP (1977) J Comput Phys 23:187
Kumar S, Bouzida D, Swendsen RH, Kollman PA, Rosenberg JM (1992) J Comput Chem 13:1011
Roux B (1995) Comput Phys Commun 91:275
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann E, Yazyev O, Austin J, Cammi R, Pomelli C, Ochterski W, Martin RL, Morokuma K, Zakrzewski VG, Voth A, Salvador P, Dannenberg JJ, Dapprich S, Daniels D, Farkas O, Foresman JB, Ortiz JV, Cioslowski, Fox J (2009) Gaussian 09, revision a.01. Gaussian, Inc, Wallingford
Lee AC, Crippen GM (2009) J Chem Inf Modl 49:2013
Davies MN, Toseland CP, Moss DS, Flower DR (2006) BMC Biochem 7:18
Olsson MHM, Sondergaard CR, Rostkowski M, Jensen JH (2011) J Chem Theo Comput 7:525
Sondergaard CR, Olsson MHM, Rostkowski M, Jensen JH (2011) J Chem Theo Comput 7:2284
Callegari D, Ranaghan KE, Woods CJ, Minari R, Tiseo M, Mor M, Mulholland AJ, Lodola A (2018) Chem Sci 9:2740
Berces A, Whitfield DM, Nukada T (2001) Tetrahedron 57:477
Liu J, Zhang C, Xu D (2012) J Mol Graph Model 37:67
Elstner M, Jalkanen KJ, Knapp-Mohammady M, Frauenheim T, Suhai S (2001) Chem Phys 263:203
Zhou H, Tajkhorshid E, Frauenheim T, Suhai S, Elstner M (2002) Chem Phys 277:91
Range K, Riccardi D, Cui Q, Elstner M, York DM (2005) Phys Chem Chem Phys 7:3070
Cui Q, Elstner M, Karplus M (2002) J Phys Chem B 106:2721
Liu J, Wang X, Xu D (2010) J Phys Chem B 114:1462
Liu J, Zheng M, Zhang C, Xu D (2013) J Phys Chem B 117:10080
Xiong J, Xu D (2017) J Phys Chem B 121:931
Sherwoord P, de Vries AH, Guest MF, Schreckenbach G, Catlow CRA, French SA, Sokol AA, Bromley ST, Thiel W, Turner AJ, Billeter S, Terstegen F, Thiel S, Kendrick J, Sc r, Casci J, Watson M, King F, Karlsen E, Sjøvoll M, Fahmi A, Schäfer A, Lennartz C (2003) J Mol Struct (THEOCHEM) 632:1
Metz S, Kästner J, Sokol AA, Keal TW, Sherwood P (2014) WIREs Comput Mol Sci 4:101
Bruice TC (2002) Acc Chem Res 35:139
Sadiq SK, Coveney PV (2015) J Chem Theory Comput 11:316
Acknowledgments
This work was funded by the National Key Research and Development Program (No. 2016YFB0700801) and the National Natural Science Foundation of China (No. 21473117). Some of the results described in this work were obtained on the Supercomputing Center of Chinese Academy of Science.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Supporting information 1
Distances between C3 of Neu5Ac unit and the carboxylate group of D315 in the CI complex (covalently sialylated enzyme intermediate); The endocyclic torsion angles for Neu5Ac unit of the CI and two products of Neu5Ac2en and Neu5Ac. B3LYP/MM single point free energy correction strategy. RMSD for the backbone atoms of the CI complex in classical MD simulation. Distance between the oxygen atom of this water molecule and C3 along the simulation time. (DOCX 1270 kb)
Rights and permissions
About this article
Cite this article
Xiong, J., Zhang, C. & Xu, D. Catalytic mechanism of type C sialidase from Streptococcus pneumoniae: from covalent intermediate to final product. J Mol Model 24, 297 (2018). https://doi.org/10.1007/s00894-018-3822-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3822-5