Summary
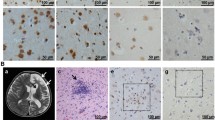
Autopsy specimens from six patients with clinically diagnosed herpes simplex virus (HSV) encephalitis were studied. Since immunocytochemistry has been reported to be more reliable and successful method to identify HSV as the etiologic agent, antitype 1 HSV (HSV1) and antitype 2 HSV (HSV2) and the avidin-biotin-peroxidase complex (ABC) method were applied to brain paraffin sections. Positive immunostaining was observed in front-orbital, mediobasal temporal lobes, cingulate gyrus, and insula. The staining was bilateral but predominant on one side. In neurons, the cytoplasmic staining was prominent in perikarya and processes, less often observed in nuclei, rarely seen in nuclear inclusions. The positive staining was intense in oligodendrocytes and macrophages, in both nuclei and cytoplasm. In two cases astrocytic processes were stained strongly. Perivascular lymphocytes were always negative. Positive reactions were obtained with both anti-HSV1 and anti-HSV2 but weaker with anti-HSV2. This results suggests that, because of its high sensitivity, ABC method permits viral antigen detection not feasible with other methods. However, this method lacks of accuracy for HSV typing mainly because of probable antigens changes resulting from tissue processing.
Similar content being viewed by others
References
Adams JH (1976) Virus diseases of the nervous system. In: Blackwood W, Consellis JAN (eds) Greenfield's neuropathology, 3rd edn. Arnold, London, pp 292–326
Aymard M (1983) Le virus herpes simplex. In: L'herpes oculaire. Bulletin des sociétés d'ophtalomologie de France. Rapport Annuel. Boissin, Marseille, pp 19–31
Baringer JR (1978) Herpes simplex infections of the nervous system. In: Vinken P, Bruyn GW (eds) Handboock of clinical neurology, vol 34: Infections of the nervous system, pt II. Elsevier, Amsterdam, pp 145–159
Budka H, Popow-Kraupp T (1981) Rabies and herpes simplex virus encephalitis. An immunohistological study on site and distribution of viral antigens. Virchows Arch [Pathol Anat] 390:353–364
Charpin C, Bhan AK, Zurawski VR, Scully R (1982) Carcinoembryonic antigen (CEA) and carbohydrate determinant (19-9) localization in 121 primary and metastatic ovarian tumors: An immunohistochemical study with the use of monoclonal antibodies. Int J Gynecol Pathol 1:231–245
Charpin C, Argemi B, Cannoni M, Oliver C, Gillioz P, Vagneur JP, Toga M (1984) Detection immunocytochimique de calcitonine, d'ACTH, de MSH, de endorphine et de somatostatine dans les carcinomes médullaires de la thyroïde. Etude en immunoperoxidase (PAP, ABC) de 10 cas. Ann Phathol 4:27–35
Chastel C (1983) Les différents pouvoirs pathogènes de l'herpes simplex virus dans L'organisme, place de l'oeil dans l'infection herpétique. In. L'herpes oculaire. Bulletin des sociétés d'ophthalmologie de France. Rapport Annuel Boissin, Marseille, pp 33–44
Chou SM, Cherry JD (1967) Ultrastructure of Cowdry type A inclusions 1. In human herpes simplex encephalitis. Neurology 17:575–586
Dinn JJ (1979) Distribution of herpes simplex virus in acute necrotising encephalitis. J Pathol 129:135–140
Esiri M (1982) Herpes simplex encephalitis. J Neurol Sci 54:209–226
Hadfield MG, Murray BK, Thomson TA, Young HF (1984) Herpes virus type 1 serum antibodies and brain tumors in humans. Clin Neuropathol 3:68–71
Hirano A (1976) Viral infection. In: An outline of neuropathology, Igaku shoin, Tokyo, pp 266–268
Hsu SM, Raine L, Fanger H (1981) The use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase techniques. J Histochem Cytochem 29:577–580
Johnson RT, Mims CA (1968) Pathogenesis of viral infections of the nervous system. N Engl J Med 278:23–30
Johnson RT (1982) Viral infections of the nervous system. Herpes virus infections. Raven Press, New York, pp 129–157
Kumanishi T, Hirano A (1978) An immunoperoxidase study on herpes simplex virus encephalitis. J Neuropathol Exp Neurol 37:790–795
Löhler J (1981) Immunohistochemical demonstration of viral antigens in paraffin embedded autopsy specimens of virally infected central nervous system. Acta Neuropathol [Suppl] (Berl) 7:139–141
Mac Callum FO, Chinn IJ, Gostling JVT (1974) Antibodies to herpes simplex virus in the cerebrospinal fluid of patients with herpetic encephalitis. J Med Microbiol 7:325–331
Meyer HM, Johnson RT, Crawford IP, Dascomb HE, Rogers NG (1960) Central nervous system syndromes of “viral” actiology. A study of 713 cases. Am J Med 29: 334–347
Miller JK, Hesser F, Tompkins VN (1965) Herpes simplex encephalitis. Ann Intern Med 64:93–103
Miyake S, Yasuraoka S, Kiryu Y, Satoyoshi E, Kurata T, Aoyama Y (1977) Herpes simplex encephalitis: an autopsy case with isolation of type 1 herpes simplex virus. Acta Neuropathol (Berl) 40:91–93
Ojeda VJ (1980) Fetal herpes simplex encephalitis with demonstration of virus in the olfactory pathway. Pathology 12:429–437
Olding-Stenkvist E (1974) Acute necrotizing herpes simplex virus encephalitis. Diagnostic problems. Scand J Infect Dis 6:355–360
Olson LC, Buescher EL, Artenstein MS, Parkman PD (1967) Herpes virus infections of the human central nervous system. New Engl J Med 277:1271–1277
Rappel M, Dubois-Dalcq M, Sprecher S, Thiry L, Lowenthal A, Pele S, Thys JP (1971) Diagnosis and treatment of herpes encephalitis. A multidisciplinary approach. J Neurol Sci 12:443–458
Rhodes RH, Novak R, Bealtie JF, West HM, Whetsell WO (1984) Immunoperoxidase demonstration of herpes simplex virus type 1 in the brain of a psychotic patient without history of encephalitis. Clin Neuropathol 3:59–67
Swoveland PT, Johnson KP (1979) Enhancement of fluorescent antibody staining of viral antigens in formalinfixed tissues by trypsin digestion. J Infect Dis 140:758–764
Toga M, Tripier MF, Payan H (1969) Encephalite aiguë nécrosante à propos de 4 observations anatomo-cliniques. Ann Pathol 14:165–184
Vestergaard BF (1973) Crossed immunoelectrophoretic characterization of herpes virus hominis type 1 and 2 antigens. Acta Pathol Mircobiol Scand [B] 81:808–810
Vestergaard BF, Grauballe PC (1977) Crossed immunoelectrophoretic identification of partially purified type common and type-specific herpes simplex virus glycoprotein antigens (39934). Proc Soc Exp Biol Med 156:349–353
Vestergaard BF, Grauballe PC (1979) Elisa for herpes simplex virus (HSV) type-specific antibodies in human sera using HSV type 1 and type 2 polyspecific antigens blocked with type heterologous rabbit antibodies. Acta Pathol Microbiol Scand [B] 87:261–263
Author information
Authors and Affiliations
Additional information
Supported by the “Fondation pour la Recherche Médicale”, “Conseil Scientifique de la Faculté de Médecine”
Rights and permissions
About this article
Cite this article
Charpin, C., Gambarelli, D., Lavaut, M.N. et al. Herpes simplex virus antigen detection in human acute encephalitis:. Acta Neuropathol 68, 245–252 (1985). https://doi.org/10.1007/BF00690202
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00690202