Summary
Two methods of determining intermediate filament protein (IFP) expression by primitive brain tumors of childhood were compared using a panel of monoclonal antibodies to three classes of IFP. In addition to a controlled immunohistochemical study, a group of these tumors was subjected to direct immunologic assay of tumor-extracted IFP using the western blot method.
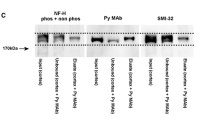
Western blots of IFP extracted from ten prospectively microdissected brain tumors revealed no NF200 or NF150 in any tumor. Traces of NF68, VFP, and GFP were detected by this sensitive method in four, three, and six cases, respectively.
Immunohistochemistry, using the same monoclonal antibodies on adjacent tumor sections, yielded results significantly different from the immunoblotting method: no NF proteins or VFP were detected, but immunoreactive GFP could be seen in a small percentage of cells in each case.
A retrospective study of 46 primitive tumors, using only immunohistochemistry, showed GFP to be the most common source of immunopositivity (38 cases), followed by VFP (15 cases), but most positive cells were judged to be reactive astrocytes. NF protein was not detected except in three cases in which extremely rare cells had morphological features of neurons. Cells which were clearly malignant, and which constituted the majority of cells in a microscopic field, were devoid of any IFP immunoreactivity.
The advantages and limitations of each method of IFP detection in this group of primitive tumors and the implications of the apparent paucity of mature neural IFP in these tumors are discussed.
Similar content being viewed by others
References
Autilio-Gambetti L, Velasco ME, Sipple J, Gambetti P (1981) Immunochemical characterization of antisera to rat neurofilament subunits. J Neurochem 37:1260–1265
Becker LE, Hinton D (1983) Primitive neuroectodermal tumors of the central nervous system. Hum Pathol 14:538–550
Bignami A, Raju T, Dahl D (1982) Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons: in vivo and in vitro immunufluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev Biol 91:286–295
Bonnin JM, Rubinstein LJ (1984) Immunohistochemistry of central nervous system tumors: its contribution to neurosurgical diagnosis. J Neurosurg 60:1121–1133
Carden MJ, Angeletti RA, Schlaepfer WW, Lee VM-Y (1984) Enzymatic dephosphorylation of neurofilaments. Soc Neurosci Abstr 10:1063
Coffin CM, Mukai K, Dehner LP (1983) Glial differentiation in medulloblastomas: histogenic insight, glial reaction or invasion of the brain? Am J Surg Pathol 7:555–565
Geisler N, Weber K (1981) Isolation of polymerization-competent vimentin from porcine eye lens tissue. FEBS Lett 125:253–256
Hinton DR, Halliday WH (1984) Primary rhabdomyosarcoma of the cerebellum. J Neuropathol Exp Neurol 43: 439–449
Houle J, Fedoroff S (1983) Temporal relationship between the appearance of vimentin and neural tube development. Dev Brain Res 9:189–195
Lazarides E (1980) Intermediate filaments as mechanical integrators of cellular space. Nature 283:249–256
Lee VM-Y, Page C, Wu H-L, Schlaepfer WW (1984) Monoclonal antibodies against gel excised glial filament proteins and their reactivity with other intermediate filament proteins. J Neurochem 42:25–32
Lee V, Wu HL, Schlaepfer WW (1982) Monoclonal antibodies recognize individual neurofilament triplet proteins. Proc Natl Acad Sci USA 79:6089–6092
Lewis SA, Balcarek JM, Krek V, Shelanski M, Cowan NJ (1984) Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: Structural conservation of intermediate filaments. Proc Natl Acad Sci USA 81:2743–2746
Mannoji H, Takeshita I, Fukui M, Ohta M, Kitamura K (1981) Glial fibrillary acidic protein in medulloblastoma. Acta Neuropathol (Berl) 55:63–69
Markesbery WR, Walsh JW, Frye MD (1980) Ultrastructural study of the medulloblastoma in tissue culture. J Neuropathol Exp Neurol 39:30–41
Marsden HB, Kumar S, Kahn J, Anderton BJ (1983) A study of glial fibrillary acidic protein in childhood brain tumors. Int J Cancer 31:439–445
Oshima RG, Howe WE, Klier FG, Adamson ED, Shevinsky LH (1983) Intermediate filament protein synthesis in pre-implant murine embryos. Dev Biol 99:447–455
Pruss RM, Mirsky R, Raff MC, Thrope R, Dowding AT, Anderton BH (1981) All clases of intermediate filaments share a common antigenic determinant defined by monoclonal antibodies. Cell 27:419–428
Quax W, Egberts WV, Hendriks W, Quax-Jeuken Y, Bloemendal H (1983) The structure of the vimentin gene. Cell 35:215–223
Roessmann U, Velasco ME, Gambetti P, Autilio-Gambetti L (1983) Neuronal and astrocytic differentiation in human neuroepithelial neoplasms: an immunohistochemical study. J Neuropathol Exp Neurol 42:113–121
Rorke LB (1983) The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol 42:1–15
Rubinstein LJ (1975) The cerebellar medulloblastoma: its origin, differentiation, morphological variants, and biological behavior. In: Vincken PJ, Bruyn GW (eds) Tumors of the brain and skull, p 3. Elsevier, New York, pp 167–193
Russell DS, Rubinstein LJ (1977) Pathology of tumors of the nervous system. Williams and Wilkins, Baltimore, p 250
Schlaepfer WW, Lee C, Trojanowski JQ, Lee YM-Y (1984) Persistence of immunoreactive neurofilament protein breakdown products in transected rat sciatic nerve. J Neurochem 43:857–864
Schlaepfer WW, Lee V, Wu H-L (1981) Assessment of immunological properties of neurofilament triplet proteins. Brain Res 226:259–272
Tapscott SJ, Bennett GS, Toyama Y, Kleinbart F, Holtzer H (1981) Intermediate filament proteins in the developing chick spinal cord. Dev Biol 86:40–54
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci 76:4350–4353
Trojanowski JQ, Obrocka MA, Lee VM-Y (1985) Distribution of neuro-filament subunits in neurons and neuronal processes: immunohistochemical studies of bovine cerebellum with subunit specific monoclonal antibodies. J Histochem Cytochem, in press
Author information
Authors and Affiliations
Additional information
Supported by grants NS 18616 (V.M.-Y.L.) and CA 36245 (J.Q.T.) from the National Institutes of Health and by the Glenn Meade Trust (G.F.T.)
Rights and permissions
About this article
Cite this article
Tremblay, G.F., Lee, V.M.Y. & Trojanowski, J.Q. Expression of vimentin, glial filament, and neurofilament proteins in primitive childhood brain tumors. Acta Neuropathol 68, 239–244 (1985). https://doi.org/10.1007/BF00690201
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00690201