Summary
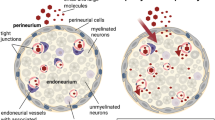
The endoneurial blood vessels of rodents are normally impermeable to proteins but they become permeable when the axons in the nerve have been severed. In this investigation, the increased permeability is examined in relation to the occurrence or absence of axonal regeneration.
The sciatic and hypoglossal nerves of rats were either ligated and transected so that axons would not regenerate, or crushed and then allowed to regenerate. Changes in vascular permeability to fluorescently labelled albumin were examined in the endoneurium distal to the sites of both types of injury at postoperative intervals of 1–21 days. When axonal regeneration was prevented, the endoneurial vessels remained impermeable to the protein tracer until the 6th day. They then became permeable throughout the distal stump and remained so for the remainder of the experimental period. When axons regenerated, there was a considerably more intense exudation of the tracer in the distal segment of the nerve. The zone of greatly increased endoneurial vascular permeability advanced along the nerve at the same rate as that of the most rapidly regenerating axons, as observed in silver-stained sections.
It is suggested that in the absence of regenerating axons, vascular permeability may be initiated by products of Wallerian degeneration. The greater permeability in regenerating nerves may be induced by vasoactive substances secreted by growth-cones. The results support a hypothesis in which it is maintained that the presence of plasma proteins around growth-cones is necessary for the occurrence of axonal regeneration. The further increase in permeability caused by the most rapidly elongating axons may assist the regenerative process by making larger quantities of plasma proteins available to other growing axons.
Similar content being viewed by others
References
Ahmed AM, Weller RO (1979) The blood-nerve barrier and reconstitution of the perineurium following nerve grafting. Neuropathol Appl Neurobiol 5:469–483
Archer GT, (1958) Release of histamine from mast cells by tissue extracts. Nature 182:726–727
Benditt EP, Arase M (1959) An enzyme in mast cells with properties like chymotrypsin. J Exp Med 110:451–460
Bissett GW, Lewis GP (1962) A spectrum of pharmacological activity in some biologically active peptides. Br J Pharmacol 19:168–182
Chapman LF, Goodell H, Wolff HG (1960) Structures and processes involved in the sensation of itch. In: Montagna W (ed) Advances in the biology of skin. Pergamon Press, Oxford, pp 161–188
Chapman LF, Ramos AO, Goodell H, Wolff HG (1961) Neurohumoral features of afferent fibers in man. Arch Neurol 4:617–650
Cockett SA (1972) A method for measuring the rate of regeneration in peripheral nerves. Exp Neurol 37:635–638
Feng TP, Gerard RW (1930) Mechanisms of nerve asphyxiation, with a note on the nerve sheath as a diffusion barrier. Proc Soc Exp Biol Med (NY) 27:1073–1076
Fukuhara N, Kumamoto T, Nakazawa Y, Tsubaki T, (1979) Bloodnerve barrier: effect of ligation of the peripheral nerve. Exp Neurol 63:573–582
Gershenbaum MR, Roisen FJ (1978) A scanning electron-microscopic study of peripheral nerve degeneration and regeneration. Neuroscience 3:1241–1250
Haftek J, Thomas PK (1968) Electron-microscope observations on the effects of localized crush injuries on the connective tissue of peripheral nerve. J Anat 103:233–243
Hasler MB (1979) The persistence and possible externalization of axonal debris during Wallerian degeneration. J Neuropathol Exp Neurol 38:242–252
Heinicke EA (1980) Vascular permeability and axonal regeneration in tissues autotransplanted to the brain. Acta Neuropathol (Berl) 49:177–185
Heinicke EA, Kiernan JA (1978) Vascular permeability and axonal regeneration in skin autotransplanted into the brain. J Anat 125:409–420
Hökfelt T, Kellerth JO, Nilsson G, Pernow B (1975a) Substance P. Localization in the central nervous system and in some primary sensory neurons. Science 190:889–890
Hökfelt T, Kellerth JO, Nilsson G, Pernow B (1975b) Experimental immunohistochemical studies on the localization of and distribution of substance P in cat primary sensory neurons. Brain Res 100:235–252
Holmes W (1943) Silver staining of nerve axons in paraffin sections. Anat Rec 86:157–187
Holton P (1959) Further observation on substance P in degenerating nerve. J Physiol 149:135P-136P
Johnson AR, Erdös EG, (1973) Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med (NY) 142:1253–1356
Kiernan JA (1972a) Effects of known and suspected neurotransmitter substances and of some nucleotides on mast cells. Experientia 28:653–655
Kiernan JA (1972b) The involvement of mast cells in vasodilatation due to axon reflexes in injured skin. Q J Exp Physiol 57:311–317
Kiernan JA (1974) Action of adenosine triphosphate on mast cells in normal and denervated skin. Arch Dermatol Forsch 251:83–86
Kiernan JA (1975) A pharmacological and histological investigation of the involvement of mast cells in cutaneous axon reflex vasodilatation. Q J Exp Physiol 60:123–130
Kiernan JA (1977) A study of chemically induced acute inflammation in the skin of the rat. Q J Exp Physiol 62:151–161
Kiernan JA (1978) An explanation of axonal regeneration in peripheral nerves and its failure in the central nervous system. Med Hypotheses 4:15–26
Kiernan JA (1979) Hypotheses concerned with axonal regeneration in the mammalian nervous system. Biol Rev 54:155–197
Kiernan JA (1981) Histological and histochemical techniques. The theory and practice. Pergamon Press, Oxford
Kiernan JA, Berry M (1975) Neuroanatomical methods. In: Bradley PB (ed) Methods in brain research, Chapt 1. Wiley, London New York, pp 1–77
Kiernan JA, Contestabile A (1980) Vascular permeability associated with axonal regeneration in the optic system of the goldfish. Acta Neuropathol (Berl) 51:39–45
Kiernan JA, Heinicke EA (1977) Estimation of lengths of regenerated axons in nerves. Exp Neurol 56:431–434
Lagunoff D (1968) The properties of mast cell proteases. Biochem Pharmacol [Suppl] 17:221–227
Mellick RS Cavanagh JB (1968) Changes in blood vessel permeability during degeneration and regeneration in peripheral nerves. Brain 91:141–160
Motte DJ de la, Allt G (1976) Crush injury to peripheral nerve. An electron microscope study employing horseradish peroxidase. Acta Neuropathol (Berl) 36:9–19
Olsson TP, Forsberg I, Kristensson K (1978) Uptake and retrograde axonal transport of horseradish peroxidase in regenerating facial motor neurons of the mouse. J Neurocytol 7:323–326
Olsson Y (1966a) Studies on vascular permeability in peripheral nerves. 1. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol (Berl) 7:1–15
Olsson Y (1966b) Studies on vascular permeability in peripheral nerves. 2. Distribution of circulating fluorescent serum albumin in rat sciatic nerve after local injection of 5-hydroxytryptamine, histamine and compound 48/80. Acta Physiol Scand [Suppl] 69:248
Olsson Y (1966c) The effect of histamine liberator compound 48/80 on mast cells in normal peripheral nerve. Acta Pathol Microbiol Scand 68:563–574
Olsson Y (1967) Phylogenetic variations in vascular permeability of peripheral nerves to serum albumin. Acta Pathol Microbiol Scand 69:621–623
Olsson Y (1968) Topographic differences in the vascular permeability of the PNS. Acta Neuropathol (Berl) 10:26–33
Olsson Y (1971) Studies on vascular permeability in peripheral nerves. 4. Distribution of intravenously injected protein tracers in the peripheral nerves of various species. Acta neuropathol (Berl) 17:114–126
Olsson Y, Kristensson K (1973) The perineurium as a diffusion barrier to protein tracers following trauma to nerves. Acta Neuropathol (Berl) 23:105–111
Olsson Y, Reese TS (1969) Inaccessibility of the endoneurium of mouse sciatic nerve to exogenous proteins. Anat Rec 163:318–319
Pernow B (1953) Studies on substance P: purification, occurrence, and biological actions. Acta Physiol Scand [Suppl] 29:1–90
Prado JL (1970) Proteolytic enzymes as kininogenases. In: Erdos EG (Hrsg) Handbuch der experimentellen Pharmakologie, vol 25. Springer, Berlin Heidelberg New York, pp 156–192
Rocha E Silva M, Reis ML, Ferreira SH (1967) Release of kinins from fresh plasma under varying experimental conditions. Biochem Pharmacol 16:1665–1676
Rowley DA, Benditt EP (1956) 5-hydroxytryptamine and histamine as mediators of the vascular injury produced by agents which damage mast cells in rats. J Exp Med 103:399–412
Shanthaveerappa TR, Bourne GM (1966) The perineural epithelium. A new concept of its role in the integrity of the peripheral nervous system. Science 154:1464–1467
Soderfelt B, Olsson Y, Kristensson K (1973) The perineurium as a diffusion barrier to protein tracers in human peripheral nerve. Acta Neuropathol (Berl) 25:120–126
Sparrow JR (1978) Uptake and retrograde transport of horseradish peroxidase (HRP) by regenerating axons. Anat Rec 190:548
Sparrow JR (1980a) Changes in vascular permeability following crushing or ligation and transection of rat sciatic nerve. Anat Rec 196:179A
Sparrow JR (1980b) Studies of neurons and regeneration. PhD Thesis, The University of Western Ontario, London, Canada
Sparrow JR, Kiernan JA (1979a) Uptake and retrograde transport of proteins by regenerating axons. Acta Neuropathol (Berl) 47:39–47
Sparrow JR, Kiernan JA (1979b) Immunocytochemical localization of plasma proteins in neuronal perikarya. Soc Neurosci Abstracts 5:63
Spector WG (1958) Substances that cause capillary permeability. Pharmacol Rev 10:475–505
Sunderland S (1978) Nerves and nerve injuries, 2nd edn. Churchill-Livingstone, London
Van Lis JMJ Jennekens FGI (1977) Plasma proteins in human peripheral nerve. J Neurol Sci 34:329–334
Windle WF (1980) Inhibition of regeneration of severed axons in the spinal cord. Exp Neurol 69:209–211
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sparrow, J.R., Kiernan, J.A. Endoneurial vascular permeability in degenerating and regenerating peripheral nerves. Acta Neuropathol 53, 181–188 (1981). https://doi.org/10.1007/BF00688020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00688020