Summary
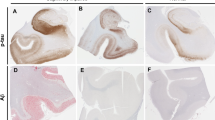
Tau immunoreactivity was studied in temporal neocortex, area 22, in 15 cases with graded intellectual status and compared with the immunoreactivity observed with an antiserum against paired helical filaments (PHF) and with the density of amyloid revealed by thioflavin S. Samples came from women over 75 years either intellectually normal or affected by senile dementia of the alzheimer type at various degrees of severity. Mental status had been prospectively assessed by the Blessed's test score. Antitau labelled a neuropil meshwork, the density of which increased with the severity of the disease. This meshwork was denser in layers II, III and V in the most affected cases. The number and the size of the taupositive fibers within the senile plaques increased with the intellectual deficit. Senile plaques were more numerous in layers II and III and neurofibrillary tangles in layers III and V whatever the staining technique: tau or PHF immunocytochemistry, and thioflavin S. The densities of senile plaques and of neurofibrillary tangles (NFT) were correlated with the severity of the disease whatever the staining method. The three methods revealed a systematically different number of changes. This systematic difference could greatly influence the neuropathological diagnosis. It could be the consequence of various factors: different sensitivities of the staining methods or changes in the antigenic and amyloid composition of the lesion according to the stage of the disease. In line with the last hypothesis, a higher proportion of amyloid-rich plaques was noted in the less affected cases, suggesting that tau and PHF epitopes appeared secondarily. Tau epitopes seemed to be present at least as early as PHF epitopes in the NFT. The pathological changes best linked to dementia were NFT revealed by tau antiserum.
Similar content being viewed by others
References
Bancher C, Lassmann H, Budka H, Grundke-Iqbal I, Iqbal K, Wiche G, Seitelberger F, Wisniewski HM (1987) Neurofibrillary tangles in Alzheimer's disease and progressive supranuclear palsy: antigenic similarities and differences. Acta Neuropathol (Berl) 74:39–46
Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM (1989). Accumulation of abnormally phosphorylated Tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res (in press)
Blessed G, Tomlinson BE, Roth M (1968) The association between quantitative measures of dementia and senile changes in the cerebral grey matter of elderly subjects. Br J Psychiatry 114:797–811
Brion JP, Couck AM, Passareiro E, Flament-Durand J (1985) Neurofibrillary tangles of Alzheimer's disease: an immunohistochemical study. J Submicrosc Cytol 17:89–96
Brion JP, Van den Bosch de Aguilar P, Flament-Durand J (1985) Senile dementia of Alzheimer type: morphological and immunocytochemical studies. In: Traber J, Gispen WH (eds) Senile dementia of Alzheimer type. Springer, Berlin Heidelberg New York Tokyo, pp 164–174
Brion JP, Passareiro E, Flament-Durand J (1986) Neurofibrillary tangles of Alzheimer's disease: ultrastructural and immunohistochemical study In: Vezzasini P, Facchini A (eds) Neuroendocrine system and aging. EURAGE, Rijswijk, pp 239–244
Delacourte A, Defossez, A (1986) Alzheimer's disease: tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J Neurol Sci 76:173–186
Duyckaerts C, Hauw J-J, Piette F, Rainsard C, Poulain V, Berthaux P, Escourolle R (1985) Cortical atrophy in senile dementia of Alzheimer type is mainly due to a decrease in cortical length. Acta Neuropathol (Berl) 66:72–74
Duyckaerts C, Hauw J-J, Bastenaire F, Piette F, Poulain V, Rainsard C, Javoy-Agid F, Berthaux P (1986) Laminar distribution of neocortical senile plaques in senile dementia of the Alzheimer type. Acta Neuropathol (Berl) 70:249–256
Duyckaerts C, Brion JP, Hauw J-J, Flament-Durant J (1987) Quantitative assessement of the density of neurofibrillary tangles and senile plaques in senile dementia of the Alzheimer type. Comparison of immunocytochemistry with a specific antibody and Bodian's protargol method. Acta Neuropathol (Berl) 73:167–170
Duyckaerts C, Delaère P, Poulain V, Brion JP, Hauw J-J (1988) Does amyloid precede paired helical filaments in the senile plaque? A study of 15 cases with graded intellectual status in aging and Alzheimer disease. Neurosci Lett 91: 354–359
Glenner GG, Wong CW (1984) Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122:1131–1135
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau, a component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Hauw J-J, Duychaerts C, Delaère P, Chaunu MP (1988) Hypothèse: maladie d'Alzheimer, amyloïde, microglie. Rev Neurol (Paris) 144:155–157
Ihara Y, Abraham C, Selkoe DJ (1983) Antibodies to paired helical filaments in Alzheimer's disease do not recognise normal brain proteins. Nature 304:727–730
Ihara Y, Nukina N, Ogawara M (1986) Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem (Tokyo) 99:1807–1810
Joachim CL, Morris JH, Phil BC, Selkoe DJ, Kosik KS (1987) Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropathol Exp Neurol 46:611–622
Kosik KS, Joachim CL, Selkoe DJ (1986) Microtubule-associated protein tau (t) is a major antigenic components of paired helical filaments in Alzheimer's disease. Proc Natl Acad Sci USA 83:4044–4048
Kowall NW, Kosik KS (1987) Axonal disruption and aberrant localization of Tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol 22:639–643
Love S, Saitoh T, Quijada S, Cole GM, Terry RD (1988) Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, Pick bodies and Lewy bodies. J Neuropathol Exp Neurol 47:393–405
Nukina N, Ihara Y (1986) One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem (Tokyo) 99:1541–1544
Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP (1985) Anatomical correlation of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82:4531–4534
Rogers J, Morrison JH (1985) Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer's disease. J Neurosci 5:2801–2808
Schwartz P (1972) Amyloidosis of the nervous system in the aged. In: Minkler J (ed) Pathology of the nervous system, vol 3. McGraw-Hill, New York, pp 2812–2849
Selkoe DJ (1987) Deciphering Alzheimer's disease: the pace quickens. Trends Neurosci 10:181–184
Snedecor OW, Cochran WO (1980) Statistical methods, 7th edn. Lowa State University Press, Ames
Sternberger LA (1979) Immunocytochemistry. Wiley, New York, pp 104–169
Wischik CM, Novak M, Thogersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A (1988) Isolation of a fragment of tau derived from the core of the paired helical fragment of Alzheimer disease. Proc Natl Acad Sci USA 85:4506–4510
Wisniewski HM, Terry RD (1973) Reexamination of the pathogenesis of senile plaques. In: Zimmerman HM (ed) Progress in neuropathology, vol 2. Grune and Straton, New York, pp 1–26
Wood JG, Mirra SS, Pollock NJ, Binder LI (1986) Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau. Proc Natl Acad Sci USA 83:4040–4043
Yen SH, Dickson DW, Crowe A, Butler M, Shelanski ML (1987) Alzheimer's neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubules-associated tau and MAP 2. Am J Pathol 126:81–91
Author information
Authors and Affiliations
Additional information
Supported by a fellowship from the European Community Commission (PD)
Rights and permissions
About this article
Cite this article
Dèlaere, P., Duyckaerts, C., Brion, J.P. et al. Tau, paired helical filaments and amyloid in the neocortex: a morphometric study of 15 cases with graded intellectual status in aging and senile dementia of Alzheimer type. Acta Neuropathol 77, 645–653 (1989). https://doi.org/10.1007/BF00687893
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687893