Summary
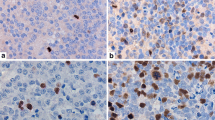
A newly developed in vitro labeling method with bromodeoxyuridine (BrdU) identifies S phase cells in situ in freshly obtained surgical tissue of human brain tumors which is subsequently fixed and embedded in paraffin for BrdU immunovisualization. For the first time, the BrdU labeling index (LI) is successfully compared here with the LI obtained by immunostaining of frozen sections of the same tumors with monoclonal antibody Ki-67 which identifies all proliferating cells, i.e., the growth fraction. LIs were counted in at least five different areas with high density of labeled cells; at least 1,000 cells were counted. In 13 metastatic tumors, Ki-67 LI was 8.3%–62.6%, and BrdU LI was 5.1%–28.0%. In 18 gliomas, Ki-67 LI was 1.4%–19.3%, and BrdU LI was 0.2%–11.6%. In 7 meningiomas, Ki-67 LI was 0.3%–3.0%, and BrdU LI was 0%–2.0%. Statistical comparison of Ki-67 and BrdU LIs by linear regression analysis revealed a highly significant correlation: BrdU LI=0.99+0.34 Ki-67 LI (r=0.92,P<0.001). A significant heterogeneity of proliferation patterns may occur within one sample from area to area, as well as between different samples of the same tumor, especially in gliomas; thus, some subjective influence on LIs by arbitrary sampling and selection could occur in quantitative evaluation of in situ cell kinetics of human brain tumors. This study indicates that our in vitro BrdU-labeling method allows the in situ identification of S phase cells in excellently preserved fixed tumor tissue which is well suited for further histological examination. This method compares favorably with Ki-67 labeling of frozen sections and might emerge as a powerful new tool for the routine study of cell proliferation in surgical specimens of human brain tumors.
Similar content being viewed by others
References
Baserga R (1981) The cell cycle. N Engl J Med 304:453–459
Bookwalter III JW, Selker RG, Schiffer L, Randall M, Iannuzzi D, Kristofik M (1986) Brain-tumor cell kinetics correlated with survival. J Neurosurg 65:795–798
Brammer I, Zywietz F, Jung H (1979) Changes of histological and proliferative indices in the Walker carcinoma with tumour size and distance from blood vessel. Eur J Cancer 15:1329–1336
Broggi G, Franzini A, Ferraresi S, Giorgi C, Allegranza A (1987) Prognostic value of labeling index in stereotactic biopsies of glial tumors. In: Chatel M, Darcel F, Pecker J (eds) Brain oncology. Martinus Nijhoff, Dordrecht, pp 75–78
Burger PC, Shibata T, Kleihues P (1986) The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology. Am J Surg Pathol 10:611–617
Gabbert H, Wagner R, Höhn P (1982) The relation between tumor cell proliferation and vascularization in differentiated and undifferentiated colon carcinomas in the rat. Virchows Arch [B] 41:119–131
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20
Gerdes J, Lemke H, Baisch H, Wacker H-N, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715
Gerdes J (1985) An immunohistological method for estimating cell growth fractions in rapid histopathological diagnosis during surgery. Int J Cancer 35:169–171
Giangaspero F, Doglioni C, Rivano MT, Pileri S, Gerdes J, Stein H (1987) Growth fraction in human brain tumors defined by the monoclonal antibody Ki-67. Acta Neuropathol (Berl) 74:179–182
Hoshino T, Wilson CB (1979) Cell kinetic analyses of human malignant brain tumors (gliomas). Cancer 44:956–962
Hoshino T (1979) The cell kinetics for gliomas: its prognostic value and therapeutic implications. Neurooncology 1: 105–112
Hoshino T, Nagashima T, Murovic J, Levin EM, Levin VA, Rupp SM (1985) Cell kinetic studies of in situ human brain tumors with bromodeoxyuridine. Cytometry 6:627–632
Hsu S-M, Raine L, Fauger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Klingerman MM, Heidenreich WF, Greene S (1962) Distribution of tritiated thymidine about capillary sinusoid in a transplanted mouse tumour. Nature 20:282–283
Landolt AM, Shibata T, Kleihues P (1987) Growth rate of human pituitary adenomas. J Neurosurg 67:803–806
Molnar P, Groothuis D, Blasberg R, Zaharko D, Owens E, Fenstermacher J (1984) Regional thymidine transport and incorporation in experimental brain and subcutaneous tumors. J Neurochem 43:421–432
Nagashima T, DeArmond SJ, Murovic J, Hoshino T (1985) Immunocytochemical demonstration of S-phase cells by anti-bromodeoxyuridine monoclonal antibody in human brain tumor tissues. Acta Neuropathol (Berl) 67:155–159
Rajewsky M (1966) Zellproliferation in normalen und malignen Geweben:3H-Thymidin-Einbau in vitro unter Standardbedingungen. Biophysik 3:65–93
Roggendorf W, Schuster T, Peiffer J (1987) Proliferative potential of meningiomas determined with the monoclonal antibody Ki-67. Acta Neuropathol (Berl) 73:361–364
Rutgers DH, Niessen DPP, Van der Linden PM (1987) Cell Kinetics of hypoxic cells in a murine tumour in vivo: flow cytometric determination of the radiation-induced blockage of cell cycle progression. Cell Tissue Kinet 20:37–42
Steel GG, Bensted JPM (1965) In vitro studies of cell proliferation in tumours. I. Critical appraisal of methods and theoretical considerations. Eur J Cancer 1:275–279
Tannock IF (1968) The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer 22:258–273
Author information
Authors and Affiliations
Additional information
Supported by funds from the Commission for Cancer Research of the Medical Faculty of the University of Vienna
Rights and permissions
About this article
Cite this article
Morimura, T., Kitz, K. & Budka, H. In situ analysis of cell kinetics in human brain tumors. Acta Neuropathol 77, 276–282 (1989). https://doi.org/10.1007/BF00687579
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687579