Summary
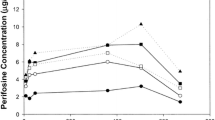
A total of 21 patients with advanced soft tissue sarcoma enrolled in a phase II trial of 3.5 g/m2 N-phosphonacetyl-l-aspartate (PALA) given intravenously every 3 weeks plus 50 mg/m2 dipyridamole (Persantine) given orally every 6 h. Dipyridamole administration was initiated 1 week before the first dose of PALA. Peak and trough plasma concentrations of dipyridamole were measured before and after the first dose of PALA in 14 patients. In all, 19 patients were evaluable for therapeutic response. One subject experienced partial regression of a pulmonary metastasis; no other major response was observed. Diarrhea was the most prominent toxicity; in one patient it was life-threatening and was associated with a severe rash. On the day preceding PALA administration, the median peak plasma concentration of dipyridamole was 2,208 ng/ml and the median trough value was 904 ng/ml. Similar values were obtained on the day of PALA administration. Although the levels achieved were similar to those required to modulate the activity of PALA in preclinical systems, the therapeutic results obtained in the present study were not superior to those reported for PALA alone in previously treated patients with soft-tissue sarcoma.
Similar content being viewed by others
References
Antman KH, Ryan L, Elias A, Sherman D, Grier HE (1989) Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J Clin Oncol 7: 126–131
Antman K, Baker L, Crowley J (1990) An intergroup (SWOG and CALGB) phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. Proc Am Soc Clin Oncol 9: 311
Baker LH, Frank J, Fine G, Balcerzak SP, Stephens RL, Stuckey WJ, Rivkin S, Saiki J, Ward JH (1987) Combination chemotherapy using Adriamycin, DTIC, cyclophosphamide, and actinomycin D for advanced soft tissue sarcomas: a randomized comparative trial. A phase III Southwest Oncology Group study (7613). J Clin Oncol 5: 851–861
Borden EC, Amato D, Rosenbaum C, Enterline HT, Shiraki MJ, Creech RH, Lerner HJ, Carbone PP (1987) Randomized comparison of three Adriamycin regimens for metastatic soft tissue sarcoma. J Clin Oncol 5: 840–850
Bramwell VHC, Mouridsen HT, Santoro A, Blackledge G, Somers R, Verwey J, Dombernowsky P, Onsurd M, Thomas D, Sylvester R, Van Oosterom A (1987) Cyclophosphamide versus ifosfamide: final report of a randomized phase II trial in adult soft tissue sarcomas. Eur J Cancer Clin Oncol 23: 311–321
Chan TCK, Young B, King ME, Taetle R, Howell S (1985) Modulation of the activity of PALA by dipyridamole. Cancer Treat Rep 69: 425–430
Chan TCK, Markman M, Cleary S, Howell SB (1986) Plasma uridine changes in cancer patients treated with the combination of dipyridamole andN-phosphonacetyl-l-aspartate. Cancer Res 46: 3168–3172
Collins KD, Stark GR (1971) Aspartate transcarbamylase, interaction with the transition state analogN-phosphonacetyl-l-aspartate. J Biol Chem 246: 6599–6605
Darnowski JW, Handschumacher RE (1985) Tissue-specific enhancement of uridine utilization and 5-fluorouracil therapy in mice by benzylacyclouridine. Cancer Res 45: 5364–5368
Darnowski JW, Handschumacher RE (1989) Enhancement of fluorouracil therapy by the manipulation of tissue uridine pools. Pharmacol Ther 41: 381–392
Erlichman C, Strong JM, Wiernick PH, McAvoy LM, Cohen MH, Levine AS, Hubbard SM, Chabner BA (1979) Phase I trial ofN-(phosphonacetyl)-l-aspartate. Cancer Res 39: 3992–3995
Ervin TJ, Blum RH, Meshad MW, Kufe DW, Johnson RK, Canellos GP (1980) Phase I trial ofN-(phosphonacetyl)-l-aspartic acid (PALA). Cancer Treat Rep 64: 1067–1071
Fischer PH, Willson JKV, Risueno C, Tutsch K, Bruggink J, Ranhosky A, Trump DL (1988) Biochemical assessment of the effects of acivicin and dipyridamole given as a continuous 72-hour intravenous infusion. Cancer Res 48: 5591–5596
Gralla RJ, Casper ES, Natale RB, Yagoda A, Young CW (1980) Phase I trial of PALA. Cancer Treat Rep 64: 1301–1305
Grem JL, Fischer PH (1986) Alteration of fluorouracil metabolism in human colon cancer cells by dipyridamole with a selective increase in fluorodeoxyuridiine monophosphate levels. Cancer Res 46: 6191–6199
Hart RD, Ohnuma T, Holland JF (1980) Initial clinical study withN-(phosphonacetyl)-l-aspartic acid (PALA) in patients with advanced cancer. Cancer Treat Rep 64: 617–624
Johnson RK (1977) Reversal of toxicity and antitumor activity ofN-(phosphonacetyl)-l-aspartate by uridine or carbamyl-dl-aspartate in vivo. Biochem Pharmacol 26: 81
Johnson RK, Inouye T, Goldin A, Stark GR (1976) Antitumor activity ofN-phosphonacetyl-l-aspartic acid, a transition-state inhibitor of aspartate transcarbamylase. Cancer Res 36: 2720
Kurzrock R, Yap B-S, Plager C, Papdopoulos N, Benjamin R, Valdivieso M, Bodey G (1984) Phase II evaluation of PALA in patients with refractory metastatic sarcomas. Am J Clin Oncol (CCT) 7: 305–307
Mahony C, Wolfram KM, Cocchetto DM, Bjornsson TD (1987) Dipyridamole kinetics. Clin Pharmacol Ther 31: 330–338
Markman M, Chan TCK, Cleary S, Howell SB (1987) Phase I trial of combination therapy of cancer withN-phosphonacetyl-l-aspartic acid and dipyridamole. Cancer Chemother Pharmacol 19: 80–83
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47: 207–214
Paterson ARP, Lau EY, Dahlig E, Cass CE (1980) A common basis of inhibition of nucleoside transport by dipyridamole and nitrobenzylthioinosine. Mol Pharmacol 18: 40
Plagemann PGW, Wohlhueter RM (1980) Permeation of nucleosides, nucleic acid bases, and nucleotides in animal cells. Curr Top Membr Transp 14: 225
Plagemann PGW, Marz R, Wohlhueter RM (1978) Uridine transport in Novikoff rat hepatoma cells and other cell lines and its relationship to uridine phosphorylation and phosphorolysis. J Cell Physiol 97: 49
Santoro A, Rouessé J, Steward W, Mouridsen H, Verweij J, Somers R, Blackledge G, Buesa J, Sayer H, Tursz T, Thomas D, Sylvester R, Van Oosterom AT (1990) A randomized EORTC study in advanced soft tissue sarcomas (STS): ADM vs ADM+IFX vs CYVADIC. Proc Am Soc Clin Oncol 9: 309
Schoenfeld DA, Rosenbaum C, Horton J, Wolter J, Falkson G, DeConti RC (1982) A comparison of Adriamycin versus vincristine and Adriamycin, versus vincristine, actinomycin-D, and cyclophosphamide for advanced sarcoma. Cancer 50: 2757–2762
Subbarao K, Rucinski B, Rausch MA, Schmid K, Niewiarowski S (1977) Binding of dipyridamole to human platelets and to α1 glycoprotein and its significance for the inhibition of adenosine uptake. J Clin Invest 60: 936–943
Valdivieso M, Moore EC, Burgess AM, Mart JR, Russ J, Plunkett W, Loo TL, Bodey GP, Freireich EJ (1980) Phase I clinical study ofN-(phosphonacetyl)-l-aspartic acid (PALA). Cancer Treat Rep 64: 285–291
Wolfram KM, Bjornsson TD (1980) High-performance liquid chromatographic analysis of dipyridamole in plasma and blood. J Chromatogr 183:57–64
Yap B-S, Baker LH, Sinkovics JG (1980) Cyclophosphamide vincristine, Adriamycin, and DTIC (CYVADIC) combination chemotherapy for the treatment of advanced sarcomas. Cancer Treat Rep 64: 93–98
Author information
Authors and Affiliations
Additional information
Supported by Boehringer Ingelheim, Inc., and NCI grant CA 47 179
Rights and permissions
About this article
Cite this article
Casper, E.S., Baselga, J., Smart, T.B. et al. A phase II trial of PALA+dipyridamole in patients with advanced soft-tissue sarcoma. Cancer Chemother. Pharmacol. 28, 51–54 (1991). https://doi.org/10.1007/BF00684956
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00684956