Abstract
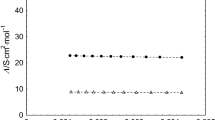
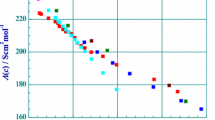
The molar conductances of lithium bromide, chloride and picrate, and of sodium picrate have been determined as a function of salt concentration in 2-propanol solvent at 25°C. Values of the limiting molar conductance, Λ0, and ion pair formation constant KA have been calculated for each of these salts using both the Fuoss 1978 and the Lee and Wheaton conductance equations. Both of these equations yield comparable results for the present systems. The limiting conductances found here are compored with those reported for lithium chloride in 1-propanol and acetone and with those for the picrates in acetone and 2-butanone, all solvents of comparable dielectric constants. The Rasaiah-Friedman square mound potential, h+−/kT, has been calculated for each salt using the approach of Justice and Justice. These values for 2-propanol have been compared with those for lithium chloride in 1-propanol and in acetone and for the picrates in acetone and 2-butanone.
Similar content being viewed by others
References
W. R. Gilkerson and A. M. Roberts,J. Am. Chem. Soc. 102, 5181 (1980).
M. K. Chantooni and I. M. Kolthoff,J. Phys. Chem. 82, 995 (1978).
R. J. Jasinski and S. Kirkland,Anal. Chem. 39, 1663 (1967).
M. A. Matesich, J. A. Nadas, and D. F. Evans,J. Phys. Chem. 74, 4568 (1970).
M. D. Jackson and W. R. Gilkerson,J. Am. Chem. Soc. 101, 328 (1979).
P. G. N. Mosely and M. Spiro,J. Solution Chem. 1, 39 (1972).
J. L. Hawes and R. L. Kay,J. Phys. Chem. 69, 2420 (1965)
R. L. Kay, B. J. Hales, and G. P. Cunningham,J. Phys. Chem. 71, 3925 (1967).
J. E. Lind, Jr., J. J. Zwolenik, and R. M. Fuoss,J. Am. Chem. Soc. 81, 1557 (1959)
W. Dannhauser and L. W. Bahe,J. Chem. Phys. 40, 3058 (1964).
R. M. Fuoss,Proc. Natl. Acad. Sci. U.S.A. 75, 16 (1978);
R. M. Fuoss,J. Phys. Chem. 82, 2427 (1978)
R. M. Fuoss,J. Solution Chem. 9, 579 (1980).
W. H. Lee and R. J. Wheaton,J. Chem. Soc. Faraday II 74, 743 (1978)
W. H. Lee and R. J. Wheaton,J. Chem. Soc. Faraday II 74, 1456 (1978)
W. H. Lee and R. J. Wheaton,J. Chem. Soc. Faraday II 75, 1128 (1979).
A. D. Pethybridge and S. S. Taba,J. Chem. Soc. Faraday I 76, 368 (1980).
See Ref. 10(b); a prepublication copy of this program was kindly supplied by R. M. Fuoss.
P. G. Wolynes,Ann. Rev. Phys. Chem. 31, 345 (1980)
J.-C. Justice and W. Ebeling,J. Solution Chem. 8, 809 (1979).
M. B. Reynolds and C. A. Kraus,J. Am. Chem. Soc. 70, 1709 (1948).
M. A. Coplan and R. M. Fuoss,J. Phys. Chem. 68, 1177 (1964).
D. F. Evans and P. Gardam,J. Phys. Chem. 73, 158 (1969).
K. Maartmann-Moe,Acta Crystallogr. Sect. B 25, 1425 (1969).
N. Bjerrum,Kgl. Danske Videnskab. 7, No. 9 (1926).
See R. M. Fuoss and C. A. Kraus,J. Am. Chem. Soc. 55, 1019 (1933), for details of these calculations.
J. C. Rasaiah and H. L. Friedman,J. Phys. Chem. 72, 3352 (1968)
J. C. Rasaiah,J. Chem. Phys. 52, 704 (1970).
J.-C. Justice and M.-C. Justice,Faraday Disc. Chem. Soc. 64, 265 (1977).
H. L. Friedman and C. V. Krishnan, inWater: A Comprehensive Treatise, Vol. III, F. Franks, ed., (Plenum Press, New York, 1973) p. 17.
D. F. Evans, J. A. Nadas, and M. S. Matesich,J. Phys. Chem. 75, 1708 (1971).
R. L. Kay,J. Am. Chem. Soc. 82, 2099 (1960).
D. F. Evans, J. Thomas, J. A. Nadas, and M. A. Matesich,J. Phys. Chem. 75, 1714 (1971).
E. Grunwald and E. Price,J. Am. Chem. Soc. 86, 4517 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feng-chun, H., Gilkerson, W.R. Ion association of lithium bromide, chloride and picrate and of sodium picrate in 2-propanol at 25°C. I. Conductance measurements. J Solution Chem 12, 161–170 (1983). https://doi.org/10.1007/BF00648054
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00648054