Summary
-
1.
The medicinal leech has five pairs of simple eyes (Fig. 1A). The photoreceptors of some eyes are known to excite one of the bilateral pair of lateral visual (LVa) cells in the first segmentai ganglion (G1). A new pair of neurons, the LVb cells, has been found in subganglion 4 (SubEG4) of the subesophageal ganglion. Since G1 and SubEG4 develop from adjacent ganglionic primordia, the LVa and LVb cells may be serial homologues.
-
2.
The somata and dendrites of the LVa and LVb cells lie in corresponding positions (Fig. 3), and their axons run side-by-side and terminate together in the supraesophageal ganglion.
-
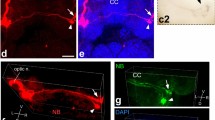
3.
Both neurons have bulbous contralateral branches (Figs. 2, 3), and fibrous ipsilateral dendrites that match the two zones where photoreceptors of the ipsilateral eyes 3–5 have synaptic terminals (Fig. 1C). Moreover, both neurons are Lucifer Yellow dye-coupled to the photoreceptors of those same eyes (Fig. 2B).
-
4.
When the ipsilateral eyes 3–5 are illuminated, the LVa and LVb cells both show a characteristic complex response that includes a compound EPSP produced by electrical coupling potentials from the photoreceptors (Figs. 5, 6), small spike-like events, and conventional-looking action potentials (Fig. 4).
-
5.
The compound EPSP is thought to elicit local spikes in ipsilateral dendritic branches, which then spread electrotonically into the main neurite and soma as small spikes. In turn, the summation of these small spikes in the neurite evidently elicits propagating action potentials.
-
6.
LV cells produce extended plateau-like depolarizations (Fig. 7) in weak or continuously varying light, or in response to current injection. These slow potentials appear to be generated by a noninactivating Na conductance and a TEA-sensitive K conductance (Fig. 8). Since slow potentials are accentuated when extracellular Ca is replaced by Ba (Fig. 9), Ca appears to participate in the response, presumably via a Ca-dependent K channel.
-
7.
Although the LVa and LVb cells may be homologous, they are not identical (Fig. 5). For example, unlike the LVa cells, the LVb cells are not inhibited by input from the contralateral eyes 3–5. Moreover, eyes 1 and 2 weakly inhibit the LVa cells, but weakly excite the LVb cells.
Similar content being viewed by others
References
Fernandez J, Stent GS (1982) Embryonic development of the hirudinid leechHirudo medicinalis: structure, development and segmentation of the germinal plate. J Embryol Exp Morphol 72:71–96
Fioravanti R, Fuortes MGF (1972) Analysis of responses in visual cells of the leech. J Physiol (Lond) 227:173–194
Frank E, Jansen JKS, Rinvik E (1975) A multisomatic axon in the central nervous system of the leech. J Comp Neurol 159:1–13
Gardner-Medwin AR, Jansen JKS, Taxt T (1973) The ‘giant’ axon of the leech. Acta Physiol Scand 87:30A-31A
Hagiwara S, Byerly L (1981) Calcium channel. Annu Rev Neurosci 4:69–125
Kretz JR, Stent GS, Kristan WB Jr (1976) Photosensory input pathways in the medicinal leech. J Comp Physiol 106:1–37
Lasansky A, Fuortes MGF (1969) The site of origin of electrical responses in visual cells of the leech,Hirudo medicinalis. J Cell Biol 42:241–252
Laverack MS (1969) Mechanoreceptors, photoreceptors and rapid conduction pathways in the leechHirudo medicinalis. J Exp Biol 50:129–140
Llinás R, Sugimori M (1980a) Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol (Lond) 305:171–195
Llinás R, Sugimori M (1980b) Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol (Lond) 305:197–213
Macagno ER (1980) Number and distribution of neurons in leech segmental ganglia. J Comp Neurol 190:283–302
Marsden CA, Kerkut GA (1969) Fluorescent microscopy of the 5-HT and catecholamine-containing cells in the central nervous system of the leechHirudo medicinalis. Comp Biochem Physiol 31:851–862
Meech RW (1978) Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng 7:1–18
Muller KJ, Carbonetto ST (1979) The morphological and physiological properties of a regenerating synapse in the C.N.S. of the leech. J Comp Neurol 185:485–516
Muller KJ, Nicholls J, Stent GS (1981) The neurobiology of the leech. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Nicholls JG, Baylor DA (1968) Specific modalities and receptive fields of sensory neurons in the CNS of the leech. J Neurophysiol 31:740–756
Nicholls JG, Purves D (1970) Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J Physiol (Lond) 209:647–667
Oertel D, Stuart AE (1981) Transformation of signals by interneurones in the barnacle's visual pathway. J Physiol (Lond) 311:127–146
Oland LA, French KA, Hayashi JH, Stuart AE (1983) Lateral visual pathway of giant barnacle. J Neurophysiol 49:516–527
Peterson EL (1983) Visual processing in the central nervous system of the leech. Nature 303:240–242
Peterson EL (1984a) The fast conducting system of the leech: a network of 93 dye-coupled interneurons. J Comp Physiol A 154:781–788
Peterson EL (1984b) Photoreceptors and visual interneurons in the medicinal leech. J Neurobiol 15:413–428
Peterson EL (1984c) Two stages of integration in a leech visual interneuron. J Comp Physiol A, 155:543–557
Peterson EL (1985a) Visual interneurons in the leech brain. II. The anterior visual cells of the supraesophageal ganglion. J Comp Physiol A 156:707–717
Peterson EL (1985b) Visual interneurons in the leech brain. III. The unpaired H cell. J Comp Physiol A 156:719–727
Röhlich P, Török LJ (1964) Elektronenmikroskopische Beobachtungen an den Sehzellen des Blutegels,Hirudo medicinalis L. Z Zellforsch 63:618–635
Stewart W (1978) Intracellular marking of neurons with a highly fluorescent naphthalimide dye. Cell 14:741–747
Tazaki K, Cooke IM (1979) Ionic bases of slow, depolarizing responses of cardiac ganglion neurons in the crab,Portunus sanguinolentus. J Neurophysiol 42:1022–1047
Yau K-W (1976) Physiological properties and receptive fields of mechanosensory neurons in the head ganglion of the leech: comparison with homologous cells in segmental ganglia. J Physiol (Lond) 263:489–512
Zipser B, McKay R (1981) Monoclonal antibodies distinguish identifiable neurones in the leech. Nature 289:549–554
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peterson, E.L. Visual interneurons in the leech brain. J. Comp. Physiol. 156, 697–706 (1985). https://doi.org/10.1007/BF00619119
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00619119