Summary
-
1.
Ascending interneurons were functionally identified and injected with Lucifer Yellow and horseradish peroxidase. The distribution of their axon terminals in the supraesophageal ganglion was examined with light and electron microscopy
-
2.
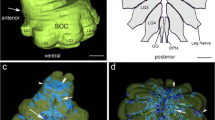
The terminals of all cells examined invade the ipsilateral parolfactory neuropil. Most of the cells also project processes to the ipsilateral antennal and/or optic neuropil and a few cells terminate in the optic ganglia. Thus ascending interneurons may distribute their actions to all three neuromeres of the brain and to distal elements of the visual pathway (Figs. 1–3).
-
3.
The axons and secondary processes of ascending interneurons are distinguished by the presence of microtubules. Both processes exhibit protuberances (Fig. 5) which in the electron microscope have all the features of subsynaptic zones (Fig. 6).
-
4.
HRP labeled processes in the synaptic neuropil region contain small round vesicles and large dense core vesicles (Fig. 7). The two vesicle types are segregated. Only the small round vesicles are found at synaptic release sites.
-
5.
Presynaptic sites containing small round vesicles (Fig. 8) are identifiable along the secondary and tertiary process of the HRP labeled ascending axon terminals. We infer that these synapses are the basis of the excitatory functional connection between ascending and descending interneurons.
-
6.
Vesicle-containing neural processes in the antennal and antennule neuropils make axo-axonic contacts with the HRP labeled ascending cell terminals (Figs. 6 and 9). A preponderance of these synapses contain elongated vesicles which imply an inhibitory function. We infer that these axoaxonic synapses are the structural correlates of the functional inhibitory inputs to ascending interneuron terminals.
-
7.
The axo-axonal synapses are principally located on the terminal arborization between the axon proper and the release sites of the ascending interneuron. This location is consistent with inhibitory action on events intermediate between the axonal action potential and the transmitter release mechanism.
Similar content being viewed by others
References
Atwood HL (1976) Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol 7:291–391
Atwood HL, Morin WA (1970) Neuromuscular and axoaxonal synapses of the crayfish opener muscle. J Ultrastruct Res 32:351–369
Atwood HL, Lang F, Morin WA (1972) Synaptic vesicles: Selective depletion in crayfish excitatory and inhibitory axons. Science 176:1353–1355
Atwood HL, Stevens JK, Marin L (1984) Axoaxonal synapse location and consequences for presynaptic inhibition in crustacean motor axon terminals. J Comp Neurol 225:64–74
Bryan JS, Krasne FB (1977) Presynaptic inhibition: The mechanism of protection from habituation of the crayfish lateral giant fibre escape response. J Physiol 271:369–390
Bunt AH (1969) Formation of coated and synaptic vesicles within neurosecretory axon terminals of the crustacean sinus gland. J Ultrastruct Res 28:411–421
Dudel J (1965) The mechanism of presynaptic inhibition at the crayfish neuromuscular junction. Pflügers Arch 284:66–80
Glantz RM (1978a) Crayfish antennal neuropil. I. Reciprocal synaptic interactions and input-output characteristics of first-order interneurons. J Neurophysiol 41:1297–1313
Glantz RM (1978b) Crayfish antennal neuropil. II. Periodic bursting elicited by sensory stimulation and extrinsic current in interneurons. J Neurophysiol 41:1314–1327
Glantz RM, Kirk MD (1980) Intercellular dye migration and electrotonic coupling within neuronal networks of the crayfish brain. J Comp Physiol 140:121–133
Glantz RM, Kirk MD, Viancour T (1981) Interneurons of the crayfish brain: The relationship between dendrite location and afferent input. J Neurobiol 12:311–320
Glantz RM, Wang-Bennett L, Waldrop B (1985) Presynaptic inhibition in the crayfish brain. I. Inhibition of a central synapse and synaptic events in presynaptic terminals. J Comp Physiol A 156:477–487
Habig C, Taylor RC (1982) The crayfish second antennae II -motoneuron structure as revealed by cobalt chloride backfilling. Comp Biochem Physiol 72A:349–358
Helm R (1928) Vergleichend-anatomische Untersuchungen über das Gehirn insbesondere das ‚Antennalganglion‘ der Dekapoden. Z Morphol Ökol Tiere 12:70–134
Kennedy D, Calabrese RL, Wine JJ (1974) Presynaptic inhibition: primary afferent depolarization in crayfish neurons. Science 186:451–454
King DG (1976) Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J Neurocytol 5:207–237
Kinnamon JC (1979) Tactile input to the crayfish tegmentary neuropile. Comp Biochem Physiol 63A:41–50
Komuro T (1981) Fine structural study of the abdominal muscle receptor organs of the crayfish (Procambarus clarkii) — Morphometrical analysis of synaptic vesicles. J Electron Microsc 30:22–28
Krasne FB, Stirling CA (1972) Synapses of crayfish abdominal ganglia with special attention to afferent and efferent connections of the lateral giant fibers. Z Zellforsch 127:526–544
Muller KJ, McMahan UJ (1976) The shapes of sensory and motor neurons and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond B 194:481–499
Nakajima Y (1971) Fine structure of the medial nucleus of the trapezoid body of the bat with special reference to two types of synaptic endings. J Cell Biol 50:121–134
Nakajima Y (1974) Fine structure of the synaptic endings on the Mauthner cell of the goldfish. J Comp Neurol 156:357–402
Nakajima Y, Tisdale AD, Henkart MP (1973) Presynaptic inhibition at inhibitory nerve terminals. A new synapse in the crayfish stretch receptor. Proc Natl Acad Sci 70:2462–2466
Otsuka M, Kravitz EA, Potter DD (1967) Physiological and chemical architecture of a lobster ganglion with particular reference to gamma-aminobutyrate and glutamate. J Neurophysiol 30:725–752
Potter DD, Landis SC, Furshpan EJ (1980) Dual function during development of rat sympathetic neurones in culture. J Exp Biol 89:57–71
Sandeman DC, Luff SE (1973) The structural organization of glomerular neuropile in the olfactory and accessory lobes of an Australian freshwater crayfish,Cherax destructor. Z Zellforsch 142:37–61
Sandeman DC, Mendum CM (1971) The structure of the central synaptic contacts on an identified crustacean motorneuron. Z Zellforsch Mikrosk Anat 119:515–525
Sandeman DC, Okajima A (1973) Statocyst-induced eye movements in the crabScylla serrata. III. The anatomical projections of sensory and motor neurons and the responses of the motor neurons. J Exp Biol 59:17–38
Sigvardt KA (1977) Sensory motor interactions in antennal reflexes of the American lobster. J Comp Physiol 118:195–214
Sutherland RM, Nunnemacher RF (1968) Microanatomy of crayfish thoracic cord and roots. J Comp Neurol 132:499–518
Tautz J, Müller-Tautz R (1983) Antennal neuropil in the brain of the crayfish: Morphology of neurons. J Comp Neurol 218:415–425
Taylor RC (1975a) Integration in the crayfish antennal neuropile: topographic representation and multiple channel coding of mechanoreceptive submodalities. J Neurobiol 6:475–499
Taylor RC (1975a) Physical and physiological properties of the crayfish antennal flagellum. J Neurobiol 6:501–519
Tisdale AN, Nakajima Y (1976) Fine structure of synaptic vesicles in two types of nerve terminals in crayfish stretch receptor organs: Influence of fixation methods. J Comp Neurol 165:369–386
Uchizono K (1965) Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature 207:642–643
Uchizono K (1967) Inhibitory synapses on the stretch receptor neurone of the crayfish. Nature 214:833–834
Wiersma CAG, Bush BMH (1963) Functional neuronal connections between the thoracic and abdominal cords of the crayfish,Procambarus clarkii (Girard). J Comp Neurol 121:207–235
Wiersma CAG, Hughes GM (1961) On the functional anatomy of neuronal units in the abdominal cord of the crayfishProcambarus clarkii (Girard). J Comp Neurol 116:209–228
Wiersma CAG, Mill PJ (1965) ‘Descending’ neuronal units in the commissure of the crayfish central nervous system; and their integration of visual, tactile and proprioceptive stimuli. J Comp Neurol 125:67–94
Wiersma CAG, Yamaguchi T (1966) The neuronal components of the optic nerve of the crayfish as studied by single unit analysis. J Comp Neurol 128:333–358
Yoshino M, Kondoh Y, Hisada M (1983) Projections of statocyst sensory neurons associated with crescent hairs in the crayfishProcambarus clarkii Girard. Cell Tissue Res 230:37–48
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang-Bennett, L.T., Glantz, R.M. Presynaptic inhibition in the crayfish brain. J. Comp. Physiol. 156, 605–617 (1985). https://doi.org/10.1007/BF00619110
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00619110