Summary
-
1.
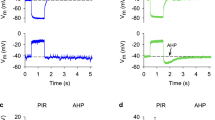
The ionic mechanism of excitability in neurosecretory cells (NSCs) of the silkworm was analysed with intrasomatic recordings, especially in comparison with surrounding non-NSCs. NSCs showed action potentials with an overshoot of 24±4 mV (mean±S.D., n = 46) and a duration of 39±11 ms at half amplitude (Fig. 1), while non NSCs showed non-overshooting spikes with an amplitude of 7.4±4.7 mV (n = 50) and a duration of 2.3±1.3 ms (Fig. 5). Action potentials of NSCs were similar in larvae, pupae and moths.
-
2.
In NSCs the action potential in the soma is primarily dependent on the presence of Ca ions, while the spike in the axon close to the soma is both Na+- and Ca++-dependent (Figs. 2, 3, 5). In non-NSCs the small spike, probably generated in the axon near the soma, is dependent only on Na+ (Fig. 5).
-
3.
The outward rectifying K conductance based on K channels is less pronounced in the NSC soma than in the non-NSC soma (Fig. 6). The latter generates Ca-dependent action potentials comparable to those in the former when K channels are blocked by TEA (Fig. 7). The excitability of the NSC soma is characterized by the less pronounced K conductance, which causes the large and prolonged, Ca-dependent action potential.
Similar content being viewed by others
Abbreviations
- NSC :
-
neurosecretory cell
- TEA :
-
tetraethylammonium
- CA :
-
corpus allatum
- SG :
-
suboesophageal ganglion
- DF :
-
cell diapause factor-producing cell
- TTX :
-
tetrodotoxin
References
Barker, J.L., Gainer, H.: Studies on bursting pacemaker potential activity in molluscan neurons. I. Membrane properties and ionic contributions. Brain Res.84, 461–477 (1975)
Barrett, E.F., Barrett, J.N.: Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurons. J. Physiol.255, 737–774 (1976)
Bassurmanova, O.K., Panov, A.A.: Structure of the neurosecretory system in Lepidoptera. Light and electron microscopy of type A'-neurosecretory cells in the brain of normal and starved larvae of the silkwormBombyx mori. Gen. Comp. Endocrinol.9, 245–262 (1967)
Bullock, T.H., Horridge, G.A.: Structure and function in the nervous systems of invertebrates. San Francisco and London: Freeman 1965
Cook, D.J., Milligan, J.V.: Electrophysiology and histology of the medial neurosecretory cells in adult male cockroaches,Periplaneta americana. J. Insect Physiol.18, 1197–1214 (1972)
Cooke, I.M.: Electrical activity of neurosecretory terminals and control of peptide hormone release. In: Peptides in neurobiology. Gainer, H. (ed.), pp. 345–374. New York, London: Plenum Press 1977
Coombs, J.S., Curtis, D.R., Eccles, J.C.: The interpretation of spike potentials of motoneurons. J. Physiol.139, 198–231 (1957)
Douglas, W.W.: Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br. J. Pharmacol. Chemother.34, 451–474 (1968)
Fatt, P., Ginsborg, B.L.: The ionic requirements for the production of action potentials in crustacean muscle fibers. J. Physiol.142, 516–543 (1958)
Fukuda, J., Kameyama, M.: Enhancement of Ca spikes in nerve cells during neurite growth in tissue culture. Nature279, 546–548 (1979)
Fukuda, S., Takeuchi, S.: Studies on the diapause factor-producing cells in the suboesophageal ganglion of the silkworm,Bombyx mori L. Embryologia9, 333–353 (1967)
Geduldig, D., Junge, D.: Sodium and calcium components of action potentials in theAplysia giant neuron. J. Physiol.199, 347–365 (1968)
Gosbee, J.L., Milligan, J.V., Smallman, B.N.: Neural responses of the protocerebral neurosecretory cells of the adult cockroach,Periplaneta americana. J. Insect Physiol.14, 1785–1792 (1968)
Hagiwara, S.: Ca spike. Adv. Biophys. (Tokyo)4, 71–102 (1973)
Hagiwara, S., Naka, K.: The initiation of spike potential in barnacle muscle fibers under low intracellular Ca++. J. Gen. Physiol.48, 141–162 (1964)
Horn, R., Miller, J.J.: A prolonged, voltage-dependent calcium permeability revealed by tetraethylammonium in the soma and axon ofAplysia giant neuron. J. Neurobiol.8, 399–415 (1977)
Hammerschlag, R., Dravid, A.R., Chiu, A.Y.: Mechanism of axonal transport: A proposed role for calcium ions. Science188, 273–275 (1975)
Hammerschlag, R., Bakhit, C., Chiu, A.Y., Dravid, A.R.: Role of calcium in the inititation of fast axonal transport of protein: effects of divalent cations. J. Neurobiol.8, 439–451 (1977)
Iwasaki, S., Satow, Y.: Sodium and calcium-dependent spike potentials in the secretory neuron soma of the X-organ of the crayfish. J. Gen. Physiol.57, 216–238 (1971)
Junge, D., Miller, J.: Different spike mechanisms in axon and soma of molluscan neuron. Nature252, 155–156 (1974)
Jungreis, A.M., Jatow, P., Wyatt, G.R.: Inorganic ion composition of haemolymph of theCecropia silkmoth: changes with diet and ontogeny. J. Insect Physiol.19, 225–233 (1973)
Kado, R.T.:Aplysia giant cell: Soma-axon voltage clamp current differences. Science182, 843–845 (1976)
Kleinhaus, A.L., Prichard, J.W.: Calcium dependent action potentials produced in leech Retzius cells by tetraethylammonium chloride. J. Physiol.246, 351–361 (1975)
Kobayashi, M.: Studies on the neurosecretion in the silkworm,Bombyx mori L. Bull. Seric. Exp. Stn.15, 181–273 (1957)
Koketsu, K., Nishi, S.: Calcium and action potentials of bullfrog sympathetic ganglion cells. J. Gen. Physiol.53, 608–628 (1969)
Krishtal, O.A., Magura, I.S.: Calcium ions as inward current carriers in molluscan neurons. Comp. Biochem. Physiol.35, 857–866 (1970)
Lux, H.D., Schubert, P., Kreutzberg, G.W., Globus, A.: Excitation and axonal flow: autoradiographic study on motoneurons intracellularly injected with a3H-amino acid. Exp. Brain Res.10, 197–204 (1970)
Mason, C.A., Bern, H.: Cellular biology of the neurosecretory neuron. In: Handbook of physiology, Sect. I, Vol. I, pp. 651–689. Bethesda, Maryland: Am. Physiol. Soc. 1977
Matsuda, Y., Yoshida, S., Yonezawa, T.: A Ca-dependent regenerative response in rodent dorsal root ganglion cells cultured in vitro. Brain Res.115, 334–338 (1976)
Meech, R.W.: Calcium-dependent potassium activation in nervous tissues. Annu. Rev. Biophys. Bioeng.7, 1–18 (1978)
Miyazaki, S.: Difference in action potentials recorded in the neurosecretory cell soma and the neuron soma in the silkworm. In: Integrative control functions of the brain. Vol. II, Ito, M. (ed.), pp. 37–38. Tokyo, Amsterdam: Kodansha Elsevier 1979
Nijhout, J.F.: Axonal pathways in the brain-retrocerebral neuro-endocrine complex ofManduca sexta (L.) (Lepidoptera: Sphingidae). Int. J. Insect Morphol. Embryol.39, 841–857 (1975)
Normann, T.C.: Membrane potential of the corpus cardiacum neurosecretory cells of the blowfly,Calliphora erythrocephala. J. Insect Physiol.19, 303–318 (1973)
Ronsom, B.R., Holz, R.W.: Ionic determination of excitability in cultured mouse dorsal root ganglion and spinal cord cells. Brain Res.136, 445–453 (1977)
Schwartzkroin, P.A., Slawsky, M.: Probable calcium spikes in hippocampal neurons. Brain Res.135, 157–161 (1977)
Sperelakis, N., Schneider, M., Harris, E.J.: Decreased K conductance produced by Ba in frog sartorius fibers. J. Gen. Physiol.50, 1565–1583 (1967)
Spitzer, N.C.: Ion channels in development. Annu. Rev. Neurosci.2, 363–397 (1979)
Takahashi, K., Miyazaki, S., Kidokoro, Y.: Development of excitability in embryonic muscle cell membranes in certain tunicates. Science171, 415–418 (1971)
Takeda, N.: The direct release of neurosecretory material from the cell in the pars intercerebralis ofMonema flavescens (Lepidoptera: Heterogeneidae). Appl. Entomol. Zool.11, 143–153 (1976)
Treherne, J.E.: The environment and function of insect nerve cells. In: Insect neurobiology. Treherne, J.E. (ed.), Frontiers of biology, Vol. 35, pp. 187–243. Amsterdam: North-Holland 1974
Wald, F.: Ionic differences between somatic and axonal action potentials in snail giant neurons. J. Physiol.220, 267–281 (1972)
Werman, R., Grundfest, H.: Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J. Gen. Physiol.44, 997–1027 (1961)
Wilkens, J.L., Mote, M.I.: Neuronal properties of the neurosecretory cells in the flySarcophaga bullata. Experientia26, 275–276 (1970)
Wyatt, S.S.: Culture in vitro of tissue from the silkworm,Bombyx mori L. J. Gen. Physiol.39, 841–857 (1956)
Author information
Authors and Affiliations
Additional information
I am indebted to Dr. A. Koike of Tochigi Prefectural Sericultural Experiment Station for guidance in the raising of silkworms and for providing material. I am grateful to Dr. E. Ohnishi, Dr. H. Ishizaki and Mr. K. Soma of Nagoya University for their advice and providing material. I also thank Dr. C. Ide for help in electron microscopy; Dr. K. Takahashi and Dr. S. Ozawa for their suggestions and comments on the manuscript and Miss Y. Hodota for typing the manuscript. — This work was supported by the grant from the Educational Ministry of Japan.
Rights and permissions
About this article
Cite this article
Miyazaki, S.i. The ionic mechanism of action potentials in neurosecretory cells and non-neurosecretory cells of the silkworm. J. Comp. Physiol. 140, 43–52 (1980). https://doi.org/10.1007/BF00613746
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00613746