Summary
-
1.
The organization of the pacemaker driving the circadian rhythm of N-acetyltransferase activity in the rat pineal gland was studied by observing changes of the rhythm caused by 1 min light pulses applied at night. These pulses proved to be effective phase-shifting signals.
-
2.
After 1 min light pulses applied in the first half of the night. N-acetyltransferase activity began to increase anew following a lag period, as if the evening rise in N-acetyltransferase were phase-delayed. After 1 min light pulses applied past midnight, N-acetyltransferase activity declined rapidly and did not increase to the high night value through the rest of night as if the morning decline in N-acetyltransferase were phase-advanced.
-
3.
The phase-response curve (PRC) showing phase-shifts of the evening N-acetyltransferase rise one day after 1 min light pulses had only phase-delays. The PRC showing phase-shifts of the morning N-acetyltransferase decline one day after the pulses had phase-advances as well as phase-delays; however the phase-advances were more pronounced.
-
4.
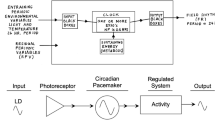
The data are consistent with a hypothesis of two-oscillator pacemaking system for the N-acetyltransferase rhythm, proposed originally by Pittendrigh and Daan (1976) for the locomotor activity rhythm in nocturnal rodents.
Similar content being viewed by others
Abbreviations
- NAT :
-
N-acetyltransferase
- PRC :
-
phase response curve
References
Aschoff J (1954) Zeitgeber der tierischen Jahresperiodik. Naturwissenschaften 41:49–59
Aschoff J (1957) Aktivitätmuster der Tagesperiodik. Naturwissenschaften 44:361–367
Boulos M, Terman M (1979) Splitting of circadian rhythms in the rat. J Comp Physiol 134:75–83
Brownstein M, Axelrod J (1974) Pineal gland. 24-h rhythm in norepinephrine turnover. Science 184:163–164
Deguchi T, Axelrod J (1972 a) Control of circadian change of serotonin N-acetyltransferase activity in the pineal organ by the beta-adrenergic receptor. Proc Natl Acad Sci USA69:2547–2550
Deguchi T, Axelrod J (1972b) Sensitive assay for serotonin Nacetyltransferase activity in rat pineal. Anal Biochem 50:174–179
Hoffmann K (1978) Photoperiodic mechanism in hamsters. The participation of the pineal gland. In: Assenmacher J, Farner DS (eds) Environmental endocrinology. Springer, Berlin Heidelberg New York, pp 94–102
Hoffmann K (1979) Photoperiodic effects in the Djungarian hamster: One minute of light during darktime mimics influence of long photoperiods on testicular recrudescence, body weight and pelage colour. Experientia (Basel) 35:1529–1530
Hoffmann K, Illnerová H, Vaněček J (1981) Effect of photoperiod and of one minute light at night-time on the pineal rhythm of N-acetyltransferase activity in the Djungarian hamsterPhodopus sungorus. Biol Reprod 24:551–556
Illnerová H, Vaněček J (1979) Response of rat pineal serotonin N-acetyltransferase to one min light pulse at different night times. Brain Res 167:431–434
Illnerová H, Bäckström M, Sääf J, Wetterberg L, Vangbo B (1978) Melatonin in rat pineal gland and serum; rapid parallel decline after light exposure at night. Neurosci Lett 9:189–193
Illnerová H, Vaněček J, Křeček J, Wetterberg L, Sääf J (1979) Effect of one minute exposure to light at night on rat pineal serotonin N-acetyltransferase and melatonin. J Neurochem 32:673–675
Klein DC, Berg GR (1970) Pineal gland: Stimulation of melatonin production by norepinephrine involves cyclic AMP-mediated stimulation of N-acetyltransferase. Adv Biochem Psychopharmacol 3:241–263
Klein DC, Moore RY (1979) Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res 174:245–262
Klein DC, Weller JE (1970) Indole metabolism in the pineal gland. A circadian rhythm in N-acetyltransferase activity. Science 169:1093–1095
Klein DC, Weller JE (1972) Rapid light-induced decrease in pineal serotonin N-acetyltransferase activity. Science 177:532–533
Klein DC, Weller JE, Moore RJ (1971) Melatonin metabolism. Neural regulation of pineal serotonin: acetylcoenzyme A N-acetyltransferase activity. Proc Natl Acad Sci USA 68:3107–3110
Moore RJ, Klein DC (1974) Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res 71:17–33
Parfitt A, Weller JL, Klein DC, Sakai KK, Marks, BH (1975) Blockade by oubain or elevated potassium ion concentration of the adrenergic and adenosine cyclic 3′,5′-monophosphate induced stimulation of pineal serotonin N-acetyltransferase activity. Mol Pharmacol 11:241–255
Pittendrigh CS (1960) Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol 25:155–184
Pittendrigh CS (1976) Circadian clocks: What are they? In: Woodward Hastings J, Schweiger HC (eds) The molecular basis of circadian rhythms. Dahlem Konferenzen, Berlin, pp 11–48
Pittendrigh CS, Daan S (1976) A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol 106:333–355
Reiter RJ (1974) Circannual reproductive rhythms in mammals related to photoperiod and pineal function. A review. Chronobiologia 1:365–395
Romero JA, Axelrod J (1975) Regulation of sensitivity to betaadrenergic stimulation in induction of pineal N-acetyltransferase. Proc Natl Acad Sci USA 72:1661–1665
Tamarkin I., Wetstrom WK, Hamill AJ, Goldman BD (1976) Effect of melatonin on the reproductive systems of male and female Syrian hamsters. A diurnal rhythm in sensitivity to melatonin. Endocrinology 99:1534–1540
Vaněček J, Illnerová H (1979) Changes of a rhythm in rat pineal serotonin N-acetyltransferase following a one-minute light pulse at night. In: Ariens Kappers J, Pevet P (eds) The pineal gland of vertebrates including man. Progress in brain research, vol 52. Elsevier/North Holland Biomedical Press, Amsterdam, pp 245–248
Vaněček J, Illnerová H (1980) Some characteristics of the night N-acetyltransferase in the rat pineal gland. J Neurochem 35:1455–1457
Weissbach H, Redfield BC, Axelrod J (1960) Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochim Biophys Acta 43:352–353
Wilkinson M, Arendt J, Bradtke J, de Ziegler D (1977) Determination of a dark-induced increase of pineal N-acetyltransferase activity and simultaneous radioimmunoassay of melatonin in pineal, serum and pituitary tissue of the male rat. J Endocrinol 72:243–244
Zatz M, Brownstein MJ (1979) Intraventricular carbachol mimics the effects of light on the circadian rhythm in the rat pineal gland. Science 203:358–361
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Illnerová, H., Vaněček, J. Two-oscillator structure of the pacemaker controlling the circadian rhythm of N-acetyltransferase in the rat pineal gland. J. Comp. Physiol. 145, 539–548 (1982). https://doi.org/10.1007/BF00612819
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00612819