Summary
-
1.
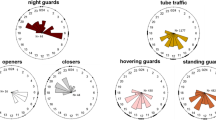
Guard bees of the stingless beeTrigona (Tetragonisca) angustula typically hover in very stable positions on both sides of and close to the nest entrance; for most of the time they face the flight corridor or the nest entrance (Fig. 2). Individual bees occupy a distinct airspace which they can leave for short excursions but return to afterwards (Fig. 3). When they change their position, they adjust their body-axis orientation to keep the nest entrance within their frontal visual field (Fig. 4). The accuracy of station-keeping decreases with the distance from the nest (Fig. 5).
-
2.
Guard bees stay tightly coupled to the nest when the whole nestbox is oscillated through 20 cm forward and sideways with respect to the direction in which the nest entrance is pointing. They hold their position and distance relative to the nest entrance by flying forward, backward and sideways while keeping the angular orientation of their body long axis constant for most of the time (Figs. 6, 7). They temporarily lag behind the nest movement when they actively change their angular orientation or when the nest moves away from them. After the movement of the nest stops, bees which have lagged behind regain hovering stations close to the nest (Fig. 8).
Similar content being viewed by others
References
Buchner E (1984) Behavioural analysis of spatial vision in insects. In: Ali MA (ed) Photoreception and vision in invertebrates. Plenum Press, New York, pp 561–621
Cartwright BA, Collett TS (1983) Landmark learning in bees. J Comp Physiol 151:521–543
Collett TS (1980) Some operating rules for the optomotor system of a hoverfly during voluntary flight. J Comp Physiol 138:271–282
Collett TS, Land MF (1975a) Visual control of flight behaviour in the hoverflySyritta pipiens L. J Comp Physiol 99:1–66
Collett TS, Land MF (1975b) Visual spatial memory in a hoverfly. J Comp Physiol 100:59–84
Egelhaaf M, Hausen K, Reichardt W, Wehrhahn C (1988) Visual course control in flies relies on neuronal computation of object and background motion. TINS 11:351–358
Gibson G (1985) Swarming behaviour of the mosquitoCulex pipiens quinquefasciatus: a quantitative analysis. Physiol Entomol 10:283–296
Götz KG (1975) The optomotor equilibrium of theDrosophila navigation system. J Comp Physiol 99:187–210
Gruhl K (1924) Paarungsgewohnheiten der Dipteren. Z Wiss Zool 122:205–280
Hengstenberg R, Sandeman DC, Hengstenberg B (1986) Compensatory head roll in the blowflyCalliphora during flight. Proc R Soc Lond B 227:455–482
Junger W, Dahmen H-J (1986) Visually induced drift compensation in waterstriders. Verh Deutsche Zool Ges 79:217
Junger W, Dahmen H-J (1988) Waterstriders (Gerridae) use visual landmarks to compensate for drift on a moving water surface. In: Elsner N, Barth FG (eds) Sense organs. Thieme, Stuttgart New York, pp 35
Koenderink JJ (1986) Optic flow. Vision Res 26:161–180
Koenderink JJ, Doom AJ van (1987) Facts on optic flow. Biol Cybern 56:247–254
Nalbach H-O, Nalbach G (1987) Distribution of optokinetic sensitivity over the eye of crabs: its relation to habitat and possible role in flow-field analysis. J Comp Physiol A 160:127–135
Preiss R (1987) Motion parallax and figural properties of depth control flight speed in an insect. Biol Cybern 57:1–9
Wehner R (1981) Spatial vision in arthropods. In: Autrum H (ed) Vision in invertebrates (Handbook of sensory physiology, vol VII 6C). Springer, Berlin Heidelberg New York, pp 287–617
Wenk P (1965) Über die Biologie blutsaugender Simuliiden (Diptera). II. Schwarmverhalten, Geschlechterfindung und Kopulation. Z Morphol Ökol Tiere 55:671–713
Wittmann D (1985) Aerial defense of the nest by workers of the stingless beeTrigona (Tetragonisca) angustula (Latreille) (Hymenoptera: Apidae). Behav Ecol Sociobiol 16:111–114
Wittmann D, Radtke R, Zeil J, Lübke G, Francke W (in press) Robberbees (Lestrimelitta limao) and their host: chemical and visual cues in nest defense byTrigona (Tetragonisca) angustula (Apidae: Meliponinae). J Chem Ecol
Zeil J (1986) The territorial flight of male houseflies (Fannia canicularis L.). Behav Ecol Sociobiol 19:213–219
Zeil J, Wittmann D (1987) Visual orientation towards the nest in a stingless bee (Tetragonisca angustula). In: Eder J, Rembold H (eds) Chemistry and biology of social insects. J Peperny, München, pp 706–707
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zeil, J., Wittmann, D. Visually controlled station-keeping by hovering guard bees ofTrigona (Tetragonisca) angustula (Apidae, Meliponinae). J. Comp. Physiol. 165, 711–718 (1989). https://doi.org/10.1007/BF00611002
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00611002