Summary
-
1.
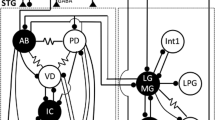
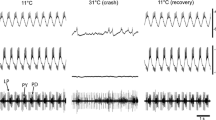
In the rock lobsterHomarus gammarus, the gastric rhythm controls the chewing movements of three cuticular teeth in the stomach (gastric mill). The rhythmic motor output (Fig. 3) arises from a network of neurones, the gastric pattern generator (GPG), located within the stomatogastric ganglion. In addition our in vitro recordings indicate that in each of the two commissural ganglia there is a gastric interneurone (CG) projecting to the stomatogastric ganglion (Fig. 4).
-
2.
The following physiological characteristics serve to identify this commissural gastric neurone (CG): (1) bursting activity phase-locked with the gastric rhythm (Fig. 4), (2) large epsps (usually larger than the decremented axon spikes) in cell body recordings (Fig. 5), (3) modulation of its activity in phase with the pyloric rhythm (Fig. 6) and (4) spontaneous spike inactivation at depolarized levels of membrane potential (Fig. 7).
-
3.
Blocking spike conduction between the stomatogastric ganglion and the commissural ganglion demonstrates that CG bursting activity is generated within the commissural ganglion (Fig. 8). Thereby this indicates that this ganglion contains a separate gastric oscillator (the commissural gastric oscillator, CGO) of which CG is an intrinsic element.
-
4.
CG itself possesses the capability for endogenous oscillation of its membrane potential (Fig. 9). This property, in association with spike inactivation at depolarized membrane potential levels, results in the ability to generate bursts of spikes either by depolarization or by hyperpolarization (Fig. 10).
-
5.
CG has strong excitatory monosynaptic connection with GM neurones of the GPG. These motor neurones are responsible for rhythmic movements of the medial tooth of the gastric mill. Furthermore activation of CG results in sequential firing of other gastric neurones, LG and LPG, responsible for movements of the two lateral teeth (Fig. 11). Due to its intrinsic properties of burstiness and spike generation within a ‘window’ of membrane potential, CG is able to activate its follower motor neurones of the GPG in several different ways (Fig. 13).
-
6.
The results suggest that the gastric motor output is organized by both a motoneuronal oscillator (the CPG) and a premotoneuronal oscillator (the CGO). This coupled oscillator organization is similar to that recently described for the pyloric system (Robertson and Moulins 1981c; Moulins and Nagy 1983). Moreover interactions between pyloric and gastric motor outputs appear to be mainly achieved at the level of the premotoneuronal oscillators of the two systems (Figs. 14–16).
Similar content being viewed by others
Abbreviations
- CPG :
-
central pattern generator
- CGO andCPO :
-
commissural gastric and pyloric oscillators
- GPG andPPG :
-
gastric and pyloric pattern generators
- CoG, OG andSTG :
-
commissural, oesophageal and stomatogastric ganglia
- CG :
-
commissural gastric interneurone
- GM :
-
medial tooth motor neurone
- LG andLPG :
-
lateral teeth motor neurones
- epsp :
-
excitatory postsynaptic potential
References
Dickinson PS, Nagy F (1983) Control of a central pattern generator by an identified modulatory interneurone in Crustacea. II. Induction and modification of plateau properties in pyloric neurones. J Exp Biol 105:59–82
Gola M, Selverston A (1981) Ionic requirements for bursting activity in lobster stomatogastric neurons. J Comp Physiol 145:191–208
Govind CK, Atwood HL, Maynard DM (1975) Innervation and neuromuscular physiology of intrinsic foregut muscles in the blue crab and the spiny lobster. J Comp Physiol 96:185–204
Grillner S (1977) On the neural control of movement. A comparison of different basic rhythm behaviors. In: Stent GS (ed) Function and formation of neural systems. Dahlem Konferenzen, Berlin, pp 197–224
Guttman R, Lewis S, Rinzel J (1980) Control of repetitive firing in squid axon membrane as a model for a neurone oscillator. J Physiol (Lond) 305:377–395
Hartline DK, Maynard DM (1975) Motor pattern in the stomatogastric ganglion of the lobsterPanulirus argus. J Exp Biol 62:405–420
Hengstenberg R (1977) Spike responses of non spiking visual interneurones. Nature 270:338–340
Kupfermann I, Weiss K (1978) The command neuron concept. Behav Brain Sci 1:3–10
Maynard DM (1972) Simpler networks. Ann NY Acad Sci B 193:59–72
Maynard DM, Dando MR (1974) The structure of the stomatogastric neuromuscular system inCallinectes sapidus, Homarus americanus andPanulirus argus. Philos Trans R Soc London Ser 268:161–220
Maynard DM, Selverston AI (1975) Organization of the stomatogastric ganglion of the spiny lobster. IV. The pyloric system. J Comp Physiol 100:161–182
Moulins M, Cournil I (1982) All-or-none control of the endogenous bursting properties of the pacemaker neurones of the lobster pyloric pattern generator. J Neurobiol 13:447–458
Moulins M, Nagy F (1981) Participation of an unpaired motor neurone on the bilaterally organized oesophageal rhythm in the lobstersJasus lalandii andPalinurus vulgaris. J Exp Biol 90:205–230
Moulins M, Nagy F (1983) Control of integration by extrinsic inputs in crustacean neuronal circuit. J Physiol (Paris) 78:755–764
Moulins M, Vedel JP (1977) Programmation centrale de l'activité motrice rhythmique du tube digestif antérieur chez les Crustacés décapodes. J Physiol (Paris) 73:471–510
Mulloney B (1977) Organization of the stomatogastric ganglion of the spiny lobster. V. Coordination of the gastric and pyloric systems. J Comp Physiol 122:227–240
Mulloney B, Selverston AI (1974a) Organization of the stomatogastric ganglion of the spiny lobster. I. Neurons driving the lateral teeth. J Comp Physiol 91:1–32
Mulloney B, Selverston AI (1974b) Organization of the stomatogastric ganglion of the spiny lobster. III. Coordination of the two subsets of the gastric system. J Comp Physiol 91:53–78
Murakami M, Shimoda Y (1977) Identification of amacrine and ganglion cells in the carp retina. J Physiol 264:180–181
Nagy F (1981) Etude de l'expression d'activités motrices rythmiques organisées par des générateurs paucineuroniques du système nerveux stomatogastrique des Crustacés décapodes. Dissertation Université de Bordeaux I
Powers L (1973) Gastric mill rhythms in intact crabs. Comp Biochem Physiol 46A:767–783
Rezer E, Moulins M (1983) Expression of the crustacean pyloric pattern generator in the intact animal. J Comp Physiol 153:17–28
Robertson RM, Moulins M (1981a) Control of rhythmic behaviour by a hierarchy of linked oscillators in Crustacea. Neurosci Lett 21:111–116
Robertson RM, Moulins M (1981b) Firing between two spike thresholds: Implications for oscillating lobster interneurons. Science 214:941–943
Robertson RM, Moulins M (1981c) Oscillatory command input to the motor pattern generators of crustacean stomatogastric ganglion. I. The pyloric rhythm. J Comp Physiol 143:453–463
Russell DF (1976) Rhythmic excitatory inputs to the lobster stomatogastric ganglion. Brain Res 101:582–588
Russel DF, Hartline DK (1978) Bursting neural networks: A re-examination. Science 200:453–456
Selverston AI (1980) Are pattern generators understandable? Behav Brain Sci 3:535–571
Selverston AI, Mulloney B (1974) Organization of the stomatogastric ganglion of the spiny lobster. II. Neurons driving the medial tooth. J Comp Physiol 91:33–51
Selverston AI, Russell DF, Miller JP, King DG (1976) The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol 7:215–289
Spirito CP (1975) The organization of the crayfish oesophageal nervous system. J Comp Physiol 102:237–249
Stein PSG (1974) The neural control of interappendage phase during locomotion. Am Zool 14:1003–1016
Tazaki K, Cooke LM (1979) Spontaneous electrical activity and interaction of large and small cells in cardiac ganglion of the crab,Portunus sanguinolentus. J Neurophysiol 42:975–999
Werblin FS (1977) Regenerative amacrine cell depolarization and formation of on-off ganglion cell response. J Physiol (Lond) 264:767–785
Wilson DM, Wyman RJ (1965) Motor output pattern during random and rhythmic stimulation of locust thoracic ganglia. Biophys J 5:121–143
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robertson, R.M., Moulins, M. Oscillatory command input to the motor pattern generators of the crustacean stomatogastric ganglion. J. Comp. Physiol. 154, 473–491 (1984). https://doi.org/10.1007/BF00610162
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610162