Summary
This paper describes the dynamics of light-evoked head reflexes in the dragonflyHemicordulia tau under light conditions which were selected to optimally address the ocelli.
-
1.
The responses occur only during flight.
-
2.
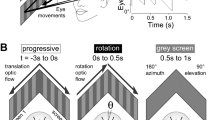
Stimulation by a light positioned to address the median ocellus evokes a head movement around the pitch axis. The threshold is in the order of 107 photons · cm−2 · s−1. With increasing intensity, the responses become progressively faster but do not increase in amplitude.
-
3.
Stimulation by lights positioned to address the lateral ocelli evokes head movements around the roll axis with a similar threshold and similar dynamics as in the pitch responses. The responses are strongest when two sources at either side of the animal are switched in alternation.
-
4.
No evidence is found for interactions between the lateral and the median inputs.
-
5.
During sustained illumination from the median source, the head is tilted towards it indefinitely, and increasing the intensity causes only a small additional change of head position. Decreasing the intensity causes a large movement of the head away from the source, and then the system readapts rapidly and the head returns to the on-position (high pass filtering). If increment pulses are superimposed on a steady background, the magnitude of their effect is a function of both their duration and amplitude.
-
6.
If the median source is modulated by a square wave of a frequency above the high pass cut-off, the amplitudes of the responses are proportional to modulation depths and independent of average intensity over 4 log units.
-
7.
At intensities below 1011 photons cm−2s−1, the spectral sensitivity has a maximum in the green, exceeding the UV-sensitivity by a factor of 5; at higher intensities the responses become more sensitive to UV than to green (reverse Purkinje shift). It is suggested that the reverse Purkinje shift is a functional adaptation to optimize the detectability of the contrast between sky and ground both in dim light and in direct sunlight.
-
8.
The dynamics of the behavioural responses can be largely accounted for by known properties of the neuronal elements of ocellar systems.
Similar content being viewed by others
References
Autrum H, Metschl N (1963) Die Arbeitsweise der Ocellen der Insekten. Z Vergl Physiol 47:256–273
Bayramoglu-Ergene S (1964) Untersuchungen über den Einfluss der Ocellen auf die Fluggeschwindigkeit der WanderheuschreckeSchistocerca gregaria. Z Vergl Physiol 48:467–480
Chappell RL, DeVoe RD (1975) Action spectra and chromatic mechanisms of cells in the median ocelli of dragonflies. J Gen Physiol 65:399–419
Chappell RL, Dowling JE (1972) Neural organisation of the median ocellus of the dragonfly. I. Intracellular electrical activity. J Gen Physiol 60:121–147
Chappell RL, Goodman LJ, Kirkham JB (1978) Lateral ocellar nerve projections in the dragonfly brain. Cell Tissue Res 190:99–114
Eaton JL (1976) Spectral sensitivity of the ocelli of the adult cabbage looper mothTrichoplusia ni. J Comp Physiol 109:17–24
Goodman LJ (1965) The role of certain optomotor reactions in regulating stability in the rolling plane during flight in the desert locust,Schistocerca gregaria. J Exp Biol 42:382–407
Goodman LJ (1970) The structure and function of the insect dorsal ocellus. Adv Insect Physiol 7:97–195
Goodman LJ (1975) The neural organization and physiology of the insect dorsal ocellus. In: Horridge GA (ed) The compound eye and vision of insects. Clarendon Press, Oxford
Guy RG, Goodman LJ, Mobbs PG (1979) Visual interneurons in the bee brain: synaptic organisation and transmission by graded potentials. J Comp Physiol 134:253–264
Hess C von (1920) Untersuchungen zur Physiologie der Stirnaugen bei Insecten. Pflügers Arch 181:1–16
Hess C von (1921) Mikroskopische Beobachtungen der phototropen Pigmentwanderung im lebenden Libellenocell. Z Biol 73:277–280
Homann H (1924a) Zum Problem der Ocellenfunktion bei den Insekten. Z Vergl Physiol 1: 541–578
Homann H (1924b) Der Vertikalilluminator als Augenspiegel bei kleinen Augen. Biol Zentralbl 44:582–591
Hu KG, Stark WS (1980) The roles ofDrosophila ocelli and compound eyes in phototaxis. J Comp Physiol 135:85–95
Kalmus H (1945) Correlations between flight and vision, and particularly between wings and ocelli in insects. Proc R Entomol Soc London A20:84–96
Kaye GWC, Laby TH (1973) Tables of physical and chemical constants. Longman, London
Kondo H (1978) Efferent system of the lateral ocellus in the dragonfly: its relationships with the ocellar afferent units, the compound eyes, and the wing sensory system. J Comp Physiol 125:341–349
Lammert A (1925) Über Pigmentwanderung im Punktauge der Insecten, sowie über Licht- und Schwerkraftreaktionen von Schmetterlingsraupen. Z Vergl Physiol 3:215–277
Laughlin SB (1980) Neural principles in the peripheral visual systems of invertebrates. In: Autrum H (ed) Handbook of sensory physiology, vol. VII/6B. Springer, Berlin Heidelberg New York
Laughlin SB, Hardie RC (1978) Common strategies for light adaptation in the peripheral visual systems of fly and dragonfly. J Comp Physiol 128:319–340
Link E (1909) Über die Stirnaugen der hemimetabolen Insekten. Zool Jahrb Anat 27:281–376
Menzel R, Knaut R (1973) Pigment movement during light and chromatic adaptation in the retinula cells ofFormica polyctena (Hymenoptera, Formicidae). J Comp Physiol 86:125–138
Metschl N (1963) Elektrophysiologische Untersuchungen an den Ocellen vonCalliphora. Z. Vergl Physiol 47:230–255
Mittelstaedt H (1950) Physiologie des Gleichgewichtssinnes bei fliegenden Libellen. Z Vergl Physiol 32:422–463
Naka KI, Rushton WAH (1966) S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185:536–555
Patterson JA, Goodman LJ (1974) Relationships between ocellar units in the ventral nerve cord and ocellar pathways in the brain ofSchistocerca gregaria. J Comp Physiol 95:251–262
Réaumur RAF de (1741) Mémoires pour servir à l'histoire des insectes, vol 5, pt 1. Mortier, Amsterdam, p 363
Rosser BL (1974) A study of the afferent pathways of the dragonfly lateral ocellus from extracellularly recorded spike discharges. J Exp Biol 60:135–160
Sandeman DC (in press) Equilibrium and proprioception systems and the central nervous system of arthropods. In: Laverack MS (ed) Proceedings of the S.E.S. Symposium on sense organs. Blackie, Glasgow
Simmons P (1980) A locust wind and ocellar brain neurone. J Exp Biol 85:281–294
Stange G, Howard J (1979) An ocellar dorsal light response in a dragonfly. J Exp Biol 83:351–355
Stavenga DG, Bernard GD, Chappell RL, Wilson M (1979) Insect pupil mechanisms. III. On the pigment migration in dragonfly ocelli. J Comp Physiol 129:199–205
Tomioka K, Yamaguchi T (1980) Steering responses of adult and nymphal crickets to light, with special reference to the head rolling movement. J Insect Physiol 26:47–57
Wilson DM (1972) Stabilizing mechanisms in insect flight. In: Proc Int Study Conf Current and future problems of acridology, London 1970. Centre for Overseas Pest Research, London, pp 47–52
Wilson M (1978a) The functional organization of locust ocelli. J Comp Physiol 124:297–316
Wilson M (1978b) Generation of graded potential signals in the second order cells of locust ocellus. J Comp Physiol 124:317–331
Wilson M (1978c) The origin and properties of discrete hyperpolarising potentials in the second order cells of locust ocellus. I Comp Physiol 128:347–358
Wyszecki G, Stiles WS (1967) Color science. Wiley, New York London Sydney, p 185
Author information
Authors and Affiliations
Additional information
I wish to thank J. Howard, S.B. Laughlin, D.C. Sandeman and M.V. Srinivasan for many helpful suggestions and for critical comments on the manuscript.
Rights and permissions
About this article
Cite this article
Stange, G. The ocellar component of flight equilibrium control in dragonflies. J. Comp. Physiol. 141, 335–347 (1981). https://doi.org/10.1007/BF00609936
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00609936