Summary
-
1.
The tonic flexor motoneurons were filled with cobalt dye via the cut ends of their axons. All six physiologically defined cells were identified anatomically (Figs. 2–4).
-
2.
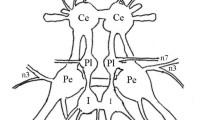
The cell somata are widely scattered in the ventral rind of the ganglia; three cells have ipsilateral and three cells have contralateral somata in reference to their axons; cells with contralateral somata tend to be more rostral in the ganglion (Figs. 2, 11).
-
3.
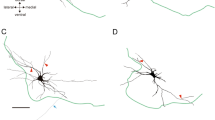
All cells have bilateral dendritic domains (Figs. 5, 6, 8, 10). Each soma is connected to a long, thin neurite which travels dorsally and enlarges into a thick process (neuropilar segment) that crosses the midline in the posterior outer commissure (Figs. 8, 9) except for fl, which crosses anteriorly (Fig. 10). Many branches emerge from the neuropilar segment; the proximal portions of these branches and the neuropilar segment contribute to the coarse dorsal neuropile. Distal branches are found in the fine ventral neuropile (Figs. 9, 10). No recurrent collaterals were observed.
-
4.
Most of the neuropilar segments converge into a narrow arc that sweeps across the dorsal neuropile (Figs. 8, 9). The close correspondence of the processes, especially of contralateral homologues (Figs. 10, 12) provides an anatomical basis for functional interactions among the tonic flexors. The main masses of finer processes that run along the longitudinal axis are located laterally.
-
5.
Bilateral, serial and interanimal homologies of soma position all showed similar degrees of variation. Positions were relatively constant but might vary by up to 100 μ relative to external landmarks (Fig. 11).
-
6.
Dendritic geometries were sufficiently similar to permit unambiguous identification of homologues, but variability in the number and shape of branches is common.
Similar content being viewed by others
References
Barker, D.L., Herbert, E., Hildebrand, J.G., Kravitz, E.A.: Acetylcholine and lobster sensory neurones. J. Physiol. (Lond.)226, 205–229 (1972)
Burrows, M.: Physiological and morphological properties of the metathoracic common inhibitory neuron of the locust. J. comp. Physiol.82, 59–78 (1973a)
Burrows, M.: The morphology of an elevator and a depressor motoneuron of the hindwing of a locust. J. comp. Physiol.83, 165–178 (1973b)
Davis, W.J.: Motoneuron morphology and synaptic contacts: Determination by intracellular dye injection. Science168, 1358–1361 (1970)
Evoy, W.H., Kennedy, D.: The central nervous organization underlying control of antagonistic muscles in the crayfish. I. Types of command fibers. J. exp. Zool.165, 223–238 (1967)
Evoy, W.H., Kennedy, D., Wilson, D.M.: Discharge patterns of neurones supplying tonic abdominal flexor muscles in the crayfish. J. exp. Biol.46, 393–411 (1967)
Florey, E.: Acetylcholine as sensory transmitter in Crustacea. New evidence from experiments demonstrating release of ACh during sensory stimulation. J. comp. Physiol.83, 1–16 (1973)
Furshpan, E.J., Potter, D.D.: Transmission at the giant motor synapses of the crayfish. J. Physiol. (Lond.)145, 289–325 (1959)
Futamachi, K.: Acetylcholine: Possible neuromuscular transmitter in Crustacea. Science175, 1373–1375 (1972)
Gillary, H.L., Kennedy, D.: Pattern generation in a crustacean motoneuron. J. Neurophysiol.32, 595–606 (1969)
Gustafson, T., Wolpert, L.: The cellular basis of morphogenesis and sea urchin development. Int. Rev. Cytol.15, 139–214 (1963)
Huxley, T.H.: The crayfish. London: C. Kegan Paul and Co., 1880
Iles, J.F., Mulloney, B.: Procion Yellow staining of cockroach motor neurones without the use of microelectrodes. Brain Res.30, 397–400 (1971)
Kendig, J.J.: Structure and function in the third abdominal ganglion of the crayfishProcambarus clarkii (Girard). J. exp. Zool.164, 1–20 (1967)
Kennedy, D.: The comparative physiology of invertebrate central neurons. Advanc. Comp. Physiol. Biochem.2, 117–184 (1966)
Kennedy, D., Evoy, W.H., Dane, B., Hanawalt, J.T.: The central nervous organization underlying control of antagonistic muscles in the crayfish. II. Coding of position by command fibers. J. exp. Zool.165, 239–248 (1967)
Kennedy, D., Evoy, W.H., Fields, H.L.: The unit basis of some crustacean reflexes. Symp. Soc. exp. Biol.20, 75–109 (1966)
Kennedy, D., Takeda, K.: Reflex control of abdominal flexor muscles in the crayfish. II. The tonic system. J. exp. Biol.43, 229–246 (1965)
Krasne, F.B., Stirling, C.A.: Synapses of crayfish abdominal ganglia with special attention to afferent and efferent connections of the lateral giant fibers. Z. Zellforsch.127, 526–544 (1972)
Kusano, K., Grundfest, H.: Circus reexcitation as a cause of repetitive activity in crayfish lateral giant axons. J. cell. comp. Physiol.65, 325–336 (1965)
Larimer, J.L., Eggleston, A.C.: Motor programs for abdominal positioning in crayfish. Z. vergl. Physiol.74, 388–402 (1971)
Nicholson, C., Llinas, R.: Field potentials in the alligator cerebellum and theory of their relationships to Purkinje cell dendritic spikes. J. Neurophysiol.34, 509–531 (1971)
Otsuka, M., Kravitz, E.A., Potter, D.D.: Physiological and chemical architecture of a lobster ganglion with particular reference to gamma-aminobutyrate and glutamate. J. Neurophysiol.30, 725–752 (1967)
Page, C.H., Sokolove, P.G.: Crayfish muscle receptor organ: Role in regulation of postural flexion. Science175, 647–650 (1972)
Pitman, R.M., Tweedle, C.D., Cohen, M.J.: Branching of central neurons: Intracellular cobalt injection for light and electron microscopy. Science176, 412–414 (1972)
Rall, W., Shepherd, G.M.: Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J. Neurophysiol.31, 884–915 (1968)
Remler, M., Selverston, A., Kennedy, D.: Lateral giant fibers of crayfish: location of somata by dye injection. Science162, 281–283 (1968)
Selverston, A.I., Remler, M.P.: Neural geometry and activation of crayfish fast flexor motoneurons. J. Neurophysiol.35, 797–814 (1972)
Stretton, A.O.W., Kravitz, E.A.: Neuronal geometry: Determination with a technique of intracellular dye injection. Science162, 132–134 (1968)
Stuart, A.E.: Physiological and morphological properties of motoneurones in the central nervous system of the leech. J. Physiol. (Lond.)209, 627–646 (1970)
Stuesse, S.L.: Chemical modification of the crayfish neuromuscular junction. Comp. and Gen. Pharmacol.4, 107–117 (1973)
Takeda, K., Kennedy, D.: Soma potentials and modes of activation of crayfish motoneurons. J. cell. Physiol.64, 165–181 (1964)
Tweedle, C.D., Pitman, R.M., Cohen, M.J.: Dendritic stability of insect central neurons subjected to axotomy and de-afferentation. Brain Res.60, 471–477 (1973)
van Harreveld, A.: A physiological solution for fresh-water crustaceans. Proc. Soc. exp. Biol. (N.Y.)34, 428–432 (1936)
Wilkens, L.A., Larimer, J.L.: Sensory interneurons: Some observations concerning the physiology and related structural significance of two cells in the crayfish brain. Tissue and Cell5, 393–401 (1973)
Zucker, R.S.: Crayfish escape behavior and central synapses. III. Electrical junctions and dendrite spikes in fast flexor motoneurons. J. Neurophysiol.35, 638–651 (1972)
Author information
Authors and Affiliations
Additional information
Supported by a grant from the Research Development Fund, Stanford University, and by NSF Grant GB-40058 to JJW, and by USPHS Grant NB-02944 to DK. We thank Grace Hagiwara for help in the preparation of figures and for technical aid, and Cecilia Bahlman, Rodney Cade, Marie Fiala, and Richard Gauthier for assistance. We are grateful to William Tatton and Phillip Sokolove for providing us with a prepublication draft of their results. Fig. 1A is drawn from a photograph provided by D. Dearmore.
Rights and permissions
About this article
Cite this article
Wine, J.J., Mittenthal, J.E. & Kennedy, D. The structure of tonic flexor motoneurons in crayfish abdominal ganglia. J. Comp. Physiol. 93, 315–335 (1974). https://doi.org/10.1007/BF00606800
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00606800