Summary
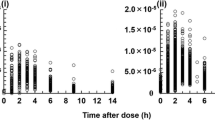
Extensive pharmacokinetic (PK) profiles after oral dosing of 300 mg cyclosporin A (CsA) were determined in whole blood by radioimmunoassay (RIA) in 14 healthy male volunteers, using two-compartment models with either first order (M1) or zero order (M0) absorption. According to zero order absorption the mean of the following PK parameters was determined: terminal half-life=12.1±5.0 h, apparent volume of distribution at steady-state=5.6 ±2.1 l · kg−1, apparent clearance=0.51±0.11 l · h−1 · kg−1. The time lag between drug ingestion and first blood level was short, 0.38±0.11 h. Drug absorption lasted for 2.8±1.6 h. The end of absorption was indicated in each individual by a sharp drop in blood levels.
The observations support the assumption that CsA is absorbed in the upper part of the small intestine with a clear-cut termination (absorption window). This assumption may explain the high degree of variability in the bioavailability of CsA.
Similar content being viewed by others
References
Atkinson K, Britton K, Paull P, Farrell C, Concannon A, Dodds A, Biggs J (1983) Detrimental effect of intestinal disease on absorption of orally administered cyclosporine. Transplant Proc 15: 2446–2449
Donatsch P, Abisch E, Homberger M, Traber R, Trapp M, Voges R (1981) A radioimmunoassay to measure cyclosporin A in plasma and serum samples. J Immunoassay 2: 19–32
Follath F, Wenk M, Vozeh S, Thiel G, Brunner F, Loertscher R, Lemaire M, Nussbaumer K, Niederberger W, Wood A (1983) Intravenous cyclosporine kinetics in renal failure. Clin Pharmacol Ther 34: 638–643
Grevel J (1986) Pharmacokinetics, metabolism and interactions of cyclosporin A. In: Ponticelli C (ed) Cyclosporin A in renal transplantation. Karger, Basel (in press)
Irschik E, Tilg H, Niederwieser D, Gash G, Huber C, Margreiter R (1984) Cyclosporin blood levels do correlate with clinical complications. Lancet 2: 692–693
Kahan BD, Wideman CA, Ried M, Gibbons S, Jarowenko M, Flechner S, van Buren CT (1984) The value of serial serum trough cyclosporine levels in human renal transplantation. Transplant Proc 16: 1195–1199
Kahan BD, Van Buren CT, Lorber MI, Flechner SM, Wideman CA, Kerman RH (1985) Optimization of cyclosporine immunosuppression for renal transplantation. Transplant Proc 17 [Suppl 2]: 35–43
Mahgoub A, Idle JR, Lancaster R, Smith RL (1977) Polymorphic hydroxylation of debrisoquine in man. Lancet 2: 584–586
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR (1985) Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther 38: 296–300
Robson S, Neuberger J, Keller HP, Abisch E, Niederberger W, von Graffenried B, Williams R (1984) Pharmacokinetic study of cyclosporin A (Sandimmune) in patients with primary biliary cirrhosis. Br J Clin Pharmacol 18: 627–631
Smith HT, Robinson WT (1984) Semi-automated high-performance liquid chromatographic method for the determination of cyclosporine in plasma and blood using column switching. J Chromatogr 305: 353–362
Ueda CT, Lemaire M, Gsell G, Nussbaumer K (1983) Intestinal lymphatic absorption of cyclosporin A following oral administration in an olive oil solution in rats. Biopharm Drug Dispos 4: 113–124
Venkataramanan R, Ptachcinski RJ, Burckhart GJ, Yang S, Starzl TE (1985) Extraction ratio of cyclosporine in a liver transplant patient with organ rejection. J Pharm Sci 74: 901–902
Wassef R, Cohen Z, Nordgren S, Langer B (1985) Cyclosporine absorption in intestinal transplantation. Transplantation 39: 496–499
Wedlund PJ, Aslanian WS, McAllister CB, Wilkinson GR, Branch RA (1984) Mephenytoin hydroxylation deficiency in Caucasians: Frequency of a new oxidative drug metabolism polymorphism. Clin Pharmacol Ther 36: 773–780
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grevel, J., Nüesch, E., Abisch, E. et al. Pharmacokinetics of oral cyclosporin a (Sandimmun) in healthy subjects. Eur J Clin Pharmacol 31, 211–216 (1986). https://doi.org/10.1007/BF00606661
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00606661